William Check, PhD
September 2015—Emerging and re-emerging viruses are well and alive, says Sherif Zaki, MD, PhD, chief of the Infectious Diseases Pathology Branch, Centers for Disease Control and Prevention. At the Clinical Virology Symposium in April, he spoke on viral etiology in unexpected deaths, presenting a list of outbreaks of unexplained illnesses in which his branch took part in the past two decades and which turned out to be caused by viruses. It was hantavirus pulmonary syndrome in the U.S. in 1993, Nipah virus in Malaysia and Singapore in 1999, SARS worldwide in 2003, MERS in the Arabian Peninsula in 2012, and others since and in between. His focus at the symposium was transplant-associated viral infections.
“Pathologists are among the first to encounter infectious disease outbreaks and are in an excellent position to recognize them,” he told attendees. “Many examples of recent emerging infectious diseases have been diagnosed through autopsies, which are increasingly being viewed as effective surveillance tools.” This was well demonstrated by one transplant-related cluster that featured what Dr. Zaki called “the persistent pathologist.”
Culture, serology, electron microscopy, immunohistochemistry, and molecular techniques have been central to finding viral causes of disease outbreaks. “Our approach to these situations depends on the clinical and epidemiologic features of the outbreak combined with histopathology,” he said. “As pathologists, we recognize patterns of inflammation—acute, chronic, granulomatous—and if there is a cytopathic effect, we can see it.”
Transmission of unusual viral pathogens from a single donor to multiple recipients of transplanted organs is not an uncommon problem, and it’s a vexing one. “We are talking about donor-derived infections, which are unexpected, unrecognized at the time of death, and not screened for in the donor,” Dr. Zaki said. “Although there is a low incidence of such events, they are high profile.”
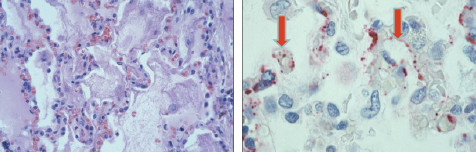
Hantavirus pulmonary syndrome. Photomicrographs of lungs: Pulmonary edema (left) and viral proteins (right). Courtesy Sherif Zaki, MD, PhD.
It is difficult to prevent these clusters for several reasons, he said: More than 25,000 transplants are done each year, 100,000 people are on waiting lists, availability of donor organs is limited, and there are few criteria for donor exclusion.
While transplant physicians have long been aware of the possibility of donor transmission of “typical” organisms, including cytomegalovirus, hepatitis B and C viruses, and Epstein-Barr virus, in the past 15 years several clusters owing to more unusual organisms have also occurred: microsporidia, lymphocytic choriomeningitis virus (LCMV), West Nile virus, and rabies. “These infections are unexpected and they attract a lot of public attention,” Dr. Zaki said, showing newspaper headlines calling into question the rules for organ transplantation.
He described investigations into clusters of transplant infections caused by lymphocytic choriomeningitis virus and rabies, highlighting the contributions of the pathologist and the laboratory.
In the first case a 51-year-old male organ recipient was found dead on the sidewalk with a subdural hematoma. Other recipients from the same donor also died. “We didn’t know the cause of death,” Dr. Zaki said. Serology for all the usual suspects was negative. It took six weeks to identify the virus as LCMV by culture, serology, histopathological examination with IHC staining, and electron microscopy. “In my opinion, these are very important tools for recognizing these diseases,” he noted.
In a second cluster, by contrast, which occurred a few years later, the patients had the same features as in the first, so investigators suspected LCMV from the outset. It took only a few days to identify the virus.
In the second case the donor was a mother who cleaned the cage of her daughter’s hamster; the cage was the source of LCMV infection. This finding underscores Dr. Zaki’s emphasis on the zoonotic nature of many clusters of unexpected illness.
Between the two LCMV incidents, seven of the eight recipients died. Both clusters were reported (Fischer SA, et al. New Engl J Med. 2006; 354:2235–2249).
“Since then we’ve had eight LCMV clusters,” Dr. Zaki said, “so something that we thought was uncommon is actually pretty common.” In one recent cluster, LCMV was identified by next-generation sequencing.
In a rabies cluster that occurred in 2004, the CDC was contacted by a pathologist in Texas about two deaths in transplant recipients and a third recipient with altered mental status, who also eventually died. The connection to a single donor came up in a fortuitous way: The patients’ families discovered it while talking in the ICU waiting room. The donor, who had a history of drug use, became ill and couldn’t swallow but was not diagnosed. The donor’s death was thought to be due to cocaine-induced brain hemorrhage.
“Our essential clue came from histopathology,” Dr. Zaki said. Staining on IHC showed Negri bodies in the brain, which are pathognomonic of rabies infection.
It is here that the “persistent pathologist” label arose. “The pathologist recalled another case of encephalitis,” Dr. Zaki said, “and went back and reviewed the autopsy.” It was consistent with encephalitis due to West Nile virus. The pathologist sent the slides to the CDC; staining showed rabies. However, this patient had received a liver from a different donor. “Was this nosocomial transmission?” Dr. Zaki asked. “Natural exposure? A second donor with rabies?”
Further investigation revealed that, while the liver came from a second donor, the artery stump to the liver came from the initial rabies-infected donor. If a surgeon doesn’t need a good artery from a donated organ, he or she may save it for another transplant, Dr. Zaki explained in a recent CAP TODAY interview. As a result, a transplant unit has “bits and pieces of tissue stored in a freezer.” In the case of the fourth rabies-infected recipient, the surgeon pulled from the freezer the artery stump from the infected donor and used it.
“A lot has changed in policies of tracking tissues and organs and it would be more difficult for this incident to happen now,” Dr. Zaki said. “They would know it was from the same donor, and if there was a problem all organs and tissues [from that donor] would be withdrawn.”
In a more recent case, a renal transplant recipient died 18 months after transplantation. “That is too long for rabies,” Dr. Zaki said. “I was betting this was not rabies.” However, Negri bodies were present in neurons of the brain, establishing the diagnosis. The donor also tested positive by PCR and histopathology. Further testing found it to be raccoon rabies, which Dr. Zaki said may explain the longer incubation period. He and his co-investigators recommended: “Rabies should be considered in patients with acute progressive encephalitis of unexplained etiology, especially for potential organ donors. A standard evaluation of potential donors who meet screening criteria for infectious encephalitis should be considered, and risks and benefits for recipients of organs from these donors should be evaluated” (Vora NM, et al. JAMA. 2013;310:398–407).
When Dr. Zaki was asked after his talk for advice on how to prevent clusters of transplant-derived infections with unusual viruses, he said, “When a person is at the door of death and they get an organ, it is hard to be picky.” Aside from routine testing for common infections and obtaining a donor exposure history, such as to pets and rodents, little can be done.
With regard to preventing transmission of lymphocytic choriomeningitis virus, Dr. Zaki tells CAP TODAY, “Unfortunately, we can’t really rely on screening donors. We can pick up some LCMV infections by serology and PCR, but most infected persons have such a low level of virus that we can’t detect it by laboratory testing.” Neither of the donors in the two clusters Dr. Zaki described tested positive for LCMV, even after that virus was identified in the recipients.
“As a pathologist, I push for the importance of donor autopsies,” Dr. Zaki said. “I feel very strongly about that. You are harvesting tissues and organs, so why not get the brain and other tissues that will allow you to test for infectious agents.” Even if a virus is found a week or two after transplantation, he noted, clinicians can be on the watch for it in the recipient. Then, too, prophylaxis, such as for rabies, may prevent illness.
To illustrate further the important part pathology plays in helping to uncover viral causes of outbreaks, Dr. Zaki presented pertinent aspects of several investigations. He begins with hantavirus pulmonary syndrome. Young people in the Four Corners region of the U.S., largely Native Americans, were dying of a flu-like illness with a high mortality rate—about 60 percent. Death, which occurred within a couple of days of onset, was caused by massive pleural effusions; on x-ray the pleural fluid showed up as a “whiteout.” “Basically they were drowning in their own secretions,” Dr. Zaki said.
Autopsy verified the presence of massive pleural effusion. “The main finding was massive amounts—six to seven liters—of pleural effusion fluid in the pleural cavity,” he said. Histopathology showed interstitial pneumonitis with fibrin and edema around alveoli. Copious amounts of immunoblasts in lymph nodes and circulating in the blood indicated an activated immune system.
“We hypothesized that the cause was plague or influenza,” Dr. Zaki said. The first clue that an unexpected pathogen was responsible came from a serology test in the special virology section at the CDC showing elevated IgG to hantavirus, suggesting that a hantavirus could be the culprit. “This finding was met with lots of skepticism,” he said, for two main reasons. First, only one strain of hantavirus was known in the Western Hemisphere, and it was not pathogenic. Second, known hantaviruses were associated with hemorrhagic disease of the kidneys, not pulmonary effusion. So hantavirus didn’t seem to fit the outbreak profile.
Proof that a hantavirus was the causative agent came from immunohistochemistry in Dr. Zaki’s laboratory and PCR in the laboratory of Stuart Nichol, PhD, chief of the CDC Viral Special Pathogens Branch. Histochemistry showed hantavirus antigens in the lungs of patients as well as the virus targeting the microcirculation in lung capillaries, causing pulmonary edema. Thus, the world learned about a new condition—hantavirus pulmonary syndrome. About two dozen strains of hantavirus are now known throughout the Western Hemisphere, many of them pathogenic.
Hantavirus deaths still occur in the U.S., he noted, citing a small outbreak in Yosemite National Park in 2012.
A second revealing outbreak investigation took place in Sarawak, Malaysia in 1997. Children ages five months to six years were dying of cardiogenic shock; EKG abnormalities were documented. Many had hand, foot, and mouth disease and many had symptoms of aseptic meningitis, leading investigators to suspect coxsackievirus myocarditis. Tissues from a dozen or so hearts were submitted to the CDC, but there was no evidence of myocarditis. “Our breakthrough came with one patient from whom we got a block from the heart and one from the CNS,” Dr. Zaki said. Review of the central nervous system tissue revealed “raging encephalitis.”
Subsequent IHC for enterovirus in brain tissue was positive, leading to identification of strain EV 71, the first time this variant had been documented to cause encephalitis. Pathogenesis was different from the initial premise. Physicians had thought that myocarditis was causing the heart problems. But the primary target was the CNS, leading to neurogenic pulmonary edema. “This shows that pathology can give clues to what is happening,” Dr. Zaki said.

Rabies transmission through solid organ transplantation, 2013. Courtesy Sherif Zaki, MD, PhD.
This syndrome continues to pop up. An outbreak occurred in Cambodia in 2012. “We still see two or three kids on the West Coast of the U.S. dying of this disease each year,” he added.
In 1998–1999 an outbreak of severe encephalitis arose in Singapore and Malaysia among those who had close contact with pigs, particularly farmers. Initially it was suspected to be due to Japanese encephalitis virus. However, electron microscopy in Dr. Zaki’s laboratory revealed the presence of paramyxovirus nucleocapsids. Further investigation showed it was a new encephalitis virus cross-reacting with the recently identified Hendra virus. The new agent was named Nipah virus. (Bats were the source.) About 1 million pigs had to be culled to break the transmission cycle.
When SARS was recognized, Cynthia Goldsmith, MS, in Dr. Zaki’s laboratory was the first to visualize the responsible coronavirus. “She was the first person to identify this virus as the cause of the outbreak,” Dr. Zaki said. The coronavirus result was quickly confirmed at the CDC by a number of other techniques, including IHC, serology, and molecular methods.
In the investigation of Middle East Respiratory Syndrome, or MERS, important information was obtained from a single autopsy of the first patient identified, in Abu Dhabi. While initial reports had characterized this patient as healthy, Dr. Zaki said the autopsy showed he was “far from healthy.” He had bad kidneys, heart disease, liver disease, and atheromas. Getting permission for an autopsy in that part of the world is particularly difficult, he said, and only considerable pressure on the government made it possible. There was a clustering of cases, which raised concern that the virus may have been transmitted by person-to-person contact, inciting a high level of fear. While the autopsy could not prove or refute this possibility (it turned out to be unfounded), it did show that healthy people are not at high risk, a suggestion borne out in subsequent cases. This remains the only autopsy done on a MERS patient.
Randall Hayden, MD, director of clinical and molecular microbiology at St. Jude Children’s Research Hospital, was a moderator for the symposium session in which Dr. Zaki spoke. Two points stand out, he said in a recent interview. “One was the role of the pathologist as one of the first lines in terms of recognizing there is something unusual and knowing how to apply appropriate methods for diagnosis or to forward specimens to someone like Dr. Zaki who has a wealth of stains and immunohistochemical techniques in his laboratory.”
The second: the availability of newer technologies such as next-generation sequencing that can complement existing technologies like immunohistochemistry “or clues from light microscopy or culture or electron microscopy.
“All of that is in the context that we are seeing more frequent emergence of new infections, so NGS will take on increasing significance for practicing pathologists and the health care system,” Dr. Hayden says.

West Nile virus (left, histopath; right, IHC). Courtesy Sherif Zaki, MD, PhD.
Of the transplant-related clusters, Dr. Hayden says: “We [at St. Jude] see patients from all over the world. We get a donor history asking whether they have been in situations where they could have been exposed. The relevance of a donor history is probably universal, but more pointed for places with immunocompromised patients and those who come from a variety of social settings.”
Vigilance—which Dr. Zaki defines as “expect the unexpected”—is the watchword to identifying and solving mysterious outbreaks, he says. “The key to . . . [detecting] a lot of these outbreaks is not just one person or one group but a combination or team effort,” he told the assembled infectious disease experts. An alert clinician, or veterinarian or pathologist, may recognize something out of the ordinary. “We all work together in these outbreaks.”
Even more important given the zoonotic linkages with many of the diseases he spoke about—hantavirus, Nipah virus, and filoviruses (the viral hemorrhagic fever agents Ebola and Marburg). It’s the One Health concept, an initiative that arose around 1999 during the outbreak of West Nile virus, Dr. Zaki says. Vital information was available from deaths of crows, but it was slow in coming to the attention of the medical community. “It became apparent that the veterinary community and the medical community were not communicating well,” he says. A government report issued in 2003 stressed the need for public health workers to collaborate with clinicians, veterinarians, and scientists in academia.
While scientists in the CDC’s Infectious Diseases Pathology Branch are often in the middle of the investigation of such viral outbreaks, there is also a role for pathologists in the community. “My message is that people in the medical community should always be aware,” Dr. Zaki says. “Sometimes you see very unusual infections associated with transplants. Everyone needs to be aware of those and know how to screen for them.” Laboratories in hospitals that perform transplants have assays for the more common viral pathogens, such as herpesvirus and adenovirus. For uncommon pathogens, one might look in the literature for information about transplant-associated infections, such as rabies, which can be diagnosed by a neuropathology exam and confirmed by special stains. “What is most important,” Dr. Zaki says, “when you become aware of an unexplained or unusual infection, is to refer such cases to a place such as CDC. The bottom line is that some diagnostic tests are only available at CDC or other specialized laboratories.”
But there and elsewhere it’s critical to remember, he says, “the frontline role of pathology” in diagnosing unexplained deaths, recognizing emerging diseases, and guiding epidemiologic investigations.
[hr]
William Check is a writer in Ft. Lauderdale, Fla.