Sherrie Rice
March 2021—If you see vascular changes, report them.
That is the advice Kristen L. Veraldi, MD, PhD, pulmonary critical care physician, University of Pittsburgh Simmons Center for Interstitial Lung Disease, gave last fall in a CAP20 virtual session on vascular changes in lung biopsies—when and what to report.
She and co-presenter Frank Schneider, MD, associate professor, Department of Pathology and Laboratory Medicine, Emory University, talked back and forth about vasculitis—what it means, what it does clinically, whether it matters.
“Is it true,” Dr. Schneider asks Dr. Veraldi, “that most patient biopsies have vascular changes that don’t matter to the patient? How often would I see a biopsy that is done for an abnormal vessel?”
Depending on the patient and clinical context, the pulmonologist will have a pretest probability for different observed findings. “If someone has a picture of significant pulmonary hypertension,” Dr. Veraldi says, “hopefully we’ve determined that already based upon exam, on diagnostic testing—for example, cardiac echo, right heart catheterization. We really aren’t sending those patients for lung biopsy. In other patients, we may be expecting to confirm a clinical suspicion of a vasculitis, in which case we should be communicating that with our pathologist in advance of the biopsy.”
The gray area, she says, is when unexpected findings are seen that the pulmonologist must explain with additional history or diagnostic testing. “We need to understand if the vascular changes are out of proportion to other findings in the biopsy, details about what the vessels look like to you, how pervasive the abnormality is.” The analogy would be understanding whether there is one tiny incidental granuloma versus sufficiently profound granulomatous change to support a diagnosis of sarcoidosis, Dr. Veraldi says.
“So we don’t necessarily need a firm diagnosis as much as a robust description of what you’re seeing so we can include that piece of the puzzle in the larger picture and develop a management plan for the patient. For example, we’ll want to make an assessment about whether these changes may herald the evolution of future clinically significant vascular disease that will impact not only our immediate diagnostics, but also inform our plan for routine monitoring and future testing.”
If pathologists see vascular changes, she says, clinicians want to know about it. “And then it’s up to us to figure out what it means.”
“I’d rather know it now than go back and identify it in retrospect in a few years when we re-review,” Dr. Veraldi says. The comment “uncertain significance—correlate clinically” is always acceptable, she adds.
Vasculitis is an inflammation of the blood vessels that, for some, means inflammation will be seen in the media, Dr. Schneider says. In reality, the intima is sometimes inflamed in blood vessels, he says. “The adventitia is also a component of the blood vessel. If you have only cuffing of the blood vessel in the adventitia by inflammatory cells, it’s probably the most tricky to decide whether there is vasculitis or not, but let’s just go with inflammation of the blood vessels,” and based on Dr. Veraldi’s advice, there should be a low threshold of noticing it and reporting it.
The small vessel vasculitides are associated with antineutrophil cytoplasmic antibodies (ANCA), with immune complexes, or with an autoimmune disease. The secondary vasculitides are caused by autoimmune diseases or malignancy or are drug induced. “And I must say that of all vasculitides I see in the lung, all comers, I see it in infections. They are very common around infections,” Dr. Schneider says.
What should pathologists look for in the medical record that would help them determine whether the vasculitis they see is significant versus an innocent bystander in another disease process? Dr. Schneider asks Dr. Veraldi.
“This is playing the ‘guess what I’m thinking’ game with your clinician,” she says. Start by looking for what serologies they’ve ordered, she advises, even if they’re not resulted yet, because that’s a clue about what the clinician is thinking. Also helpful are the data from the cardiac echo or right heart catheterization. “And if we’ve done a good job of documenting our thought process, then we may have discussed in our clinical differential what we’re thinking about, what’s highest on the list, what’s lower on the list, why we’ve decided to pursue biopsy,” Dr. Veraldi says.
If that’s not clearly expressed in the clinical notes, talk to the clinician. Time doesn’t always permit, but even a quick email sometimes can help fill in some of the blanks, she says. “Hopefully, we’ve done a pretty good job of leaving that bread crumb trail in our documentation and with the studies that we’ve ordered leading up to that biopsy for you to figure out what we’re thinking about.”
The 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides (Fig. 1) divides vasculitides into three categories, and its purpose, Dr. Schneider says, is to lead everyone, when a set of changes is seen in the vessels, to call it the same thing. “None of these changes are necessarily specific for any particular disease. Many are defined by the presence or absence of necrotizing vasculitis and the presence or absence of immune complexes or of ANCAs. But the point is that the main disease process with the large vessel vasculitis will be in a large vessel.”

That doesn’t mean it can’t involve an intraparenchymal vessel, Dr. Schneider says. “Sometimes you wonder if you see transient arteritis in a larger pulmonary artery. Hopefully you won’t see those in a peripheral wedge biopsy, but it’s a possibility. As with polyarteritis nodosa, it is more of a medium vessel vasculitis.” But what is usually seen in the lung, he says, is a full spectrum of ANCA-associated vasculitides, which, in this classification, are divided into three types.
“If you see a vasculitis that involves one of these vessels or one certain caliber vessel, should I as a pathologist be able to put that name on it and diagnose it? And I think the answer to that is no.”
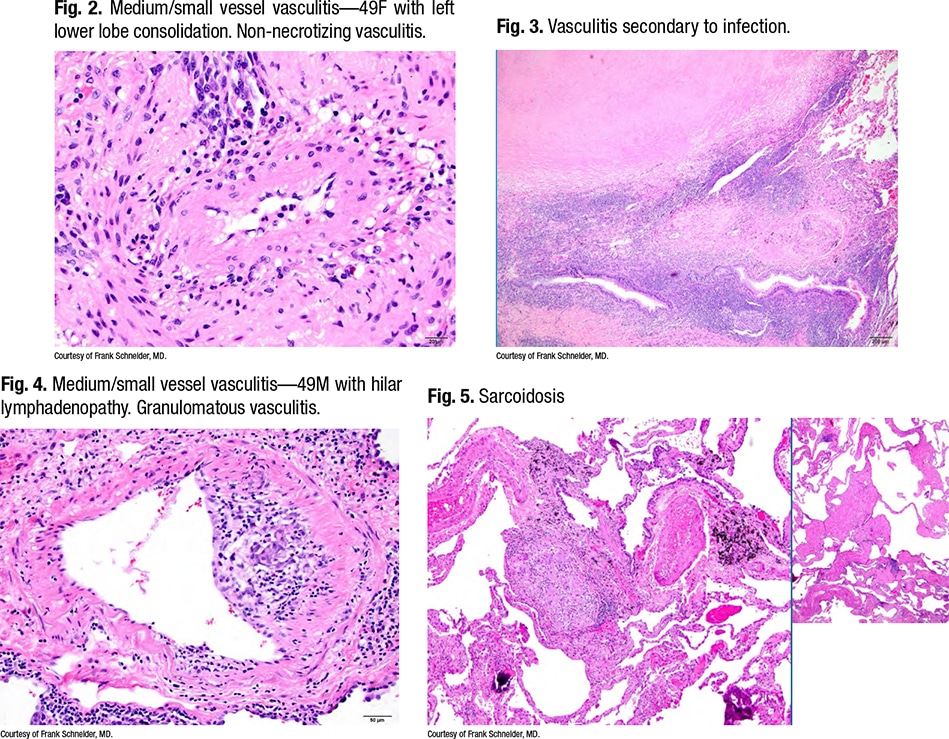
Dr. Schneider points to an image of a medium/small vessel vasculitis (Fig. 2) taken from a 49-year-old woman who had a left lower lobe consolidation. Seen on the slide was a non-necrotizing vasculitis. “What you notice is that this vasculitis is set in a bronchovascular bundle that is immediately adjacent to a very large necrotizing granuloma which, to me, looks like an infectious granuloma. It has a very pale pink, fine granular necrosis, and indeed this ended up having Histoplasma organisms in it.” (Fig. 3).
There is vasculitis next to a granuloma, he says, noting it’s common for infectious disease lung biopsies to have secondary vasculitis, “as an innocent bystander.”
 “As a pathologist, I always wonder: Could this patient have two diseases—both fleas and lice, as it’s often said?”
“As a pathologist, I always wonder: Could this patient have two diseases—both fleas and lice, as it’s often said?”
He asks Dr. Veraldi: How often do you see patients who have a necrotizing granuloma and then coincidentally have a coexisting systemic vasculitis syndrome that would have been missed had it not been reported?
“It’s not common,” Dr. Veraldi says, and the hope is that this possibility would have been considered in advance. “On rare occasions we’re surprised by microbiology results from surgical tissue if our history didn’t suggest risk factors for more uncommon infections.” She recalls a case in which she and colleagues had difficulty deciding whether a patient had granulomatosis with polyangiitis (GPA, Wegener’s) or if the lung abnormalities were entirely driven by infection. “Our patients are always allowed to have both. Ultimately, in this case, we ended up making the diagnosis of, yes, fleas and lice. This is someone who was treated for her Histoplasma and then ended up with persistent abnormalities that supported a true diagnosis of GPA.”
 Before considering immunosuppression, she and colleagues will always err on the side of treating infection and collaboration with infectious disease colleagues if there’s any doubt. “So, overall, relatively uncommon as a new diagnosis from a surgical biopsy, and if we’re hunting for infection on transbronchial biopsies, hopefully we’ve communicated this with you in advance,” she says of the pathologist. Dr. Schneider says he therefore would report this as a necrotizing granuloma “and say there is focally a little bit of vasculitis that I presume is secondary to the infection.”
Before considering immunosuppression, she and colleagues will always err on the side of treating infection and collaboration with infectious disease colleagues if there’s any doubt. “So, overall, relatively uncommon as a new diagnosis from a surgical biopsy, and if we’re hunting for infection on transbronchial biopsies, hopefully we’ve communicated this with you in advance,” she says of the pathologist. Dr. Schneider says he therefore would report this as a necrotizing granuloma “and say there is focally a little bit of vasculitis that I presume is secondary to the infection.”
Fig. 4 is a granulomatous vasculitis in a patient with sarcoidosis (Fig. 5). “The important feature is that this kind of fibrosis and the granulomatous infection follows lymphangitic routes,” Dr. Schneider says, “so it’s a characteristic distribution. And it turns out that on wedge biopsies, probably 60, 70 percent of all sarcoid biopsies have vasculitis in them.” He generally doesn’t comment on them.
“Should I be commenting on this?” he asks Dr. Veraldi. Does she treat sarcoidosis patients with vasculitis differently than sarcoidosis patients without vasculitis?
 “We don’t,” she says. “We make decisions about routine monitoring and the need for systemic immunosuppression—whether to start it and what medications to use—based on the overall clinical picture and not just the biopsy findings.”
“We don’t,” she says. “We make decisions about routine monitoring and the need for systemic immunosuppression—whether to start it and what medications to use—based on the overall clinical picture and not just the biopsy findings.”
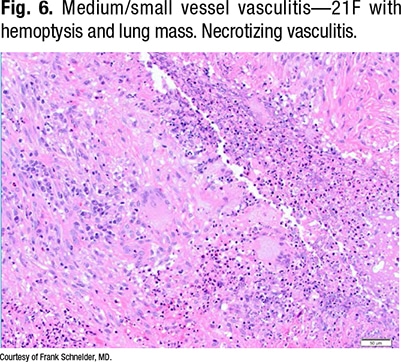
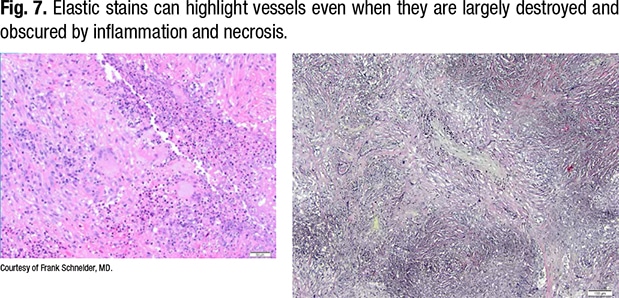
Fig. 6 is a 21-year-old with hemoptysis and a lung mass. “There’s a necrotizing vasculitis, and if someone showed that to me, I would ask them to prove to me that it’s a vasculitis,” Dr. Schneider says. “All I see is necrosis and some giant cells, so it’s something granulomatous with vasculitis, and that’s where your elastic stains come in.”
In Fig. 7, on the right of the same field (lower power), the outline of the vessel can be seen. The lumen is obliterated by organization, and the internal elastic of the vessel can be seen.
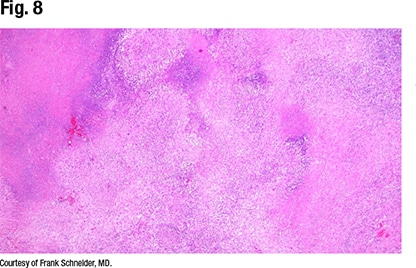
“This is a vessel completely destructed by a necrotizing vasculitis with some granulomatous features, some giant cells, around it. This is a little more necrotizing-looking than a sarcoid biopsy, and sure enough, when you look around, you see the characteristic features of GPA,” Dr. Schneider says (Fig. 8).
Fig. 9 is another occluded vessel, completely surrounded and destructed. Organizing pneumonia is seen, especially in the periphery. “There’s a pure organizing pneumonia variant of GPA, but it’s uncommon. But surrounding these masses you often have a lot of organizing pneumonia, and you have to be aware of that if you see a giant cell, maybe a little bit of vasculitis, and get a core biopsy maybe just from the periphery of a lesion like this,” Dr. Schneider says.
 Features of GPA are seen in Fig. 9, so it can be diagnosed as GPA, which Dr. Schneider says he does. “I sometimes describe it as necrotizing vasculitis with parenchymal geographic necrosis and neutrophilic microabscesses and giant cells consistent with GPA . . . though it doesn’t really matter how you describe it. But sometimes, what I find a little disturbing, I see those features and there is no ANCA. If there was no ANCA testing, I don’t know how that changes the post-test probability, but if there was an ANCA test and it was negative,” that raises the question: Can GPA be diagnosed in a patient without ANCA?
Features of GPA are seen in Fig. 9, so it can be diagnosed as GPA, which Dr. Schneider says he does. “I sometimes describe it as necrotizing vasculitis with parenchymal geographic necrosis and neutrophilic microabscesses and giant cells consistent with GPA . . . though it doesn’t really matter how you describe it. But sometimes, what I find a little disturbing, I see those features and there is no ANCA. If there was no ANCA testing, I don’t know how that changes the post-test probability, but if there was an ANCA test and it was negative,” that raises the question: Can GPA be diagnosed in a patient without ANCA?
Yes, says Dr. Veraldi. “If ANCA is negative, it’s not going to dissuade us from the diagnosis if all of the other pieces of the puzzle fit. It’s been estimated that about 10 to 20 percent of patients with GPA are ANCA negative at the time of diagnosis, which, unfortunately, means that sometimes GPA is overlooked, particularly in those patients with nonspecific findings. So it’s very helpful for us if you comment on that”—GPA can be seen in patients without ANCA—“in your report because not everyone who’s going to read the pathology report will understand that you can have GPA in the setting of ANCA negativity.” This is part of what contributes to the delayed diagnosis, she says.
Fig. 10 could be called vasculitis because there is infiltration of the media by mostly eosinophils and the vessels are surrounded by eosinophils. “If you zoom out [Fig. 11] and dig into the history,” Dr. Schneider says, “you realize this patient had pneumothorax 10 days ago and got a chest tube; because he had a persistent air leak, at some point the thoracic surgeon moved in and stapled over that persistent air leak, so you get a little wedge biopsy.”
 The fibroblast foci that are seen are not specific for usual interstitial pneumonia. “You can see them in these reactive processes,” he says. “You can see parenchymal eosinophilia not just in the pleura of pneumothorax patients, but also deeper in the subpleural lung and especially around vessels. That makes it look like an eosinophilic vasculitis. That does not mean you are dealing with a patient who has eosinophilic granulomatosis with polyangiitis, what we used to call Churg-Strauss syndrome.”
The fibroblast foci that are seen are not specific for usual interstitial pneumonia. “You can see them in these reactive processes,” he says. “You can see parenchymal eosinophilia not just in the pleura of pneumothorax patients, but also deeper in the subpleural lung and especially around vessels. That makes it look like an eosinophilic vasculitis. That does not mean you are dealing with a patient who has eosinophilic granulomatosis with polyangiitis, what we used to call Churg-Strauss syndrome.”
Fig. 12 is a medium/small vessel vasculitis in a man who has asthma and patchy lung infiltrates. “This looks surprisingly similar. There are fewer eosinophils than we saw in the previous case, but you see a slightly eccentric involvement of the small vessel by eosinophils. I would not call this necrotizing at this point, but it’s clearly there and it’s worth commenting on.” And this was a patient with eosinophilic granulomatosis with polyangiitis (Fig. 13).
Dr. Schneider recalls a professor from his medical school days many years ago saying that Churg-Strauss is essentially Wegener in a patient who has asthma. What other clue, if eosinophils are seen around vessels, should prompt the pathologist to make the diagnosis? And should the pathologist make the diagnosis or not? he asks Dr. Veraldi.
 “The pathology is going to be one part of a larger puzzle we have to piece together,” she says. Most important to her is that the pathologist describe in words as thoroughly as possible what he or she sees so she can understand what the pattern is and can put it into context. “I’d much rather you give me a highly descriptive paragraph than a one-sentence diagnosis,” Dr. Veraldi says.
“The pathology is going to be one part of a larger puzzle we have to piece together,” she says. Most important to her is that the pathologist describe in words as thoroughly as possible what he or she sees so she can understand what the pattern is and can put it into context. “I’d much rather you give me a highly descriptive paragraph than a one-sentence diagnosis,” Dr. Veraldi says.
With these patients, rheumatologists often get involved, Drs. Schneider and Veraldi note, and the perivascular infiltration by eosinophils is only one of several criteria they use to diagnose eosinophilic granulomatosis with polyangiitis.
Dr. Schneider says he sometimes worries that if he puts vasculitis in his report, testing that costs the patient thousands of dollars in copays will follow—“a multimillion dollar workup for what might be a spurious finding or incidental finding or a nonconsequential finding in a lung biopsy.” If a pathologist reports vasculitis, he asks Dr. Veraldi, what is your next step?
Different physicians will have different approaches based on their personal experience, she says, “but it fundamentally goes back to the theme of the company it keeps.
 “I need to decide if what you describe is expected or not. If it’s unexpected, then I have to comb through my other data and what I’ve been thinking to date to figure out my next steps for diagnosis and management. If I’m truly puzzled by the finding, it’s important that I can reach out to you to review the slides together and discuss the case in more detail.” Even better, she says, would be a multidisciplinary conference so the data in aggregate can be discussed and a consensus formulated.
“I need to decide if what you describe is expected or not. If it’s unexpected, then I have to comb through my other data and what I’ve been thinking to date to figure out my next steps for diagnosis and management. If I’m truly puzzled by the finding, it’s important that I can reach out to you to review the slides together and discuss the case in more detail.” Even better, she says, would be a multidisciplinary conference so the data in aggregate can be discussed and a consensus formulated.
“For people who aren’t fortunate enough to have that support,” Dr. Veraldi says, “I think we all have a large network of people we call upon when we need to phone a friend to discuss a case and make sure we’re being thoughtful about incorporating your description, your words, into our thought process. But it won’t cost the patient a small fortune. By the time we get to the biopsy, we have usually already done an exhaustive workup.”
The small vessel vasculitides are often nonspecific and not a sign of either ANCA-associated immune complex disease or the medium vessel vasculitis like polyarteritis nodosa, Dr. Schneider sums up. But do report them, because they are either a piece of the puzzle today or will develop significance over time, and clinicians are careful not to overinterpret them.
A more meaningful diagnosis, he says, is the inflammation that involves the smallest of vessels, the capillaries in the lungs, known as capillaritis. This is the damage at the alveolar level, and once the thin capillary is destroyed, it typically leads to alveolar hemorrhage, called diffuse alveolar hemorrhage. Dr. Schneider spoke about how to recognize it and whether it matters.
The histopathologic features of diffuse alveolar hemorrhage are fibrin, red blood cells, and hemosiderin-laden macrophages.
Alveolar hemorrhage can be patchy and that’s characteristic, Dr. Schneider says (Fig. 14). “The tricky part is distinguishing this from the typical procedural hemorrhage you see in nearly every wedge biopsy, because there’s always some blood vessel damage that floods the alveoli with blood.” It’s good to have a high threshold for diagnosing diffuse alveolar hemorrhage and not calling every blood in the alveoli hemorrhage, he says, because roughly more than 90 percent of the blood seen in wedge biopsies is procedure related. “I always try to hold out for all three features,” Dr. Schneider says, unless the pre-test probability is different.
One feature to be reminded of, he says, can be prominent in patients who have chronic bleeds (Fig. 15). “You see what we call ‘endogenous pneumoconiosis,’ which is a misnomer. This has nothing to do with pneumoconiosis.” In chronic bleeding, the iron from the blood will start encrusting the fragmented elastic fibers of destructed vessels and then get phagocytized by giant cells and histiocytes. “You see these iron-encrusted elastic fibers and smaller pieces around.”
Capillaritis is one cause of pulmonary hemorrhage. What should pathologists expect to see if they get a biopsy for it?
Pathologists and pulmonologists alike appreciate that there’s a broad differential for pulmonary hemorrhage, and capillaritis is one etiology, though that is the finding that requires a biopsy for confirmation or exclusion, Dr. Veraldi says. “We’re going to have a pretty good idea of whether we suspect capillaritis or not based on clinical presentation, based on our findings on bronchoscopy. The bronchoscopy itself, and to a certain extent the thoracic imaging, will lead us toward whether we think this is truly diffuse alveolar hemorrhage versus another source of airway bleeding with subsequent secondary aspiration of blood.”
 The etiology can extend from the airway to the parenchyma, she says, and be driven by other clinically recognized syndromes—is the patient on anticoagulation, for example, or does the patient have substance abuse history? As some of the known etiologies are excluded, “we end up, as with other lung diseases, in the idiopathic category,” Dr. Veraldi says. “But if we’re doing a biopsy, generally our question is, do we see capillaritis? And the clinical presentation is diffuse alveolar hemorrhage.” Pulmonary capillaritis is usually the consequence of an underlying immune-mediated process that is systemic in nature, she says, though on rare occasions, pulmonary capillaritis occurs in isolation. “Clinically, hemoptysis is not present in all patients with diffuse alveolar hemorrhage, so its absence does not exclude the need for bronchoscopy with serial lavage to confirm or exclude if it’s clinically suspected.”
The etiology can extend from the airway to the parenchyma, she says, and be driven by other clinically recognized syndromes—is the patient on anticoagulation, for example, or does the patient have substance abuse history? As some of the known etiologies are excluded, “we end up, as with other lung diseases, in the idiopathic category,” Dr. Veraldi says. “But if we’re doing a biopsy, generally our question is, do we see capillaritis? And the clinical presentation is diffuse alveolar hemorrhage.” Pulmonary capillaritis is usually the consequence of an underlying immune-mediated process that is systemic in nature, she says, though on rare occasions, pulmonary capillaritis occurs in isolation. “Clinically, hemoptysis is not present in all patients with diffuse alveolar hemorrhage, so its absence does not exclude the need for bronchoscopy with serial lavage to confirm or exclude if it’s clinically suspected.”
Identifying capillaritis in the setting of diffuse alveolar hemorrhage is important, she says, because it will have an impact on management decisions. Eighty-eight percent of patients with DAH have capillaritis, Dr. Schneider says (Travis WD, et al. Am J Surg Pathol. 1990;14[12]:1112–1125).
With serial lavage, do the tubes get redder or paler as you go? he asks.
“The important feature here is to do the lavage properly, and this is pulmonary 101, learned in fellowship. You want to wedge in a segment and stay wedged as you do your serial lavage,” Dr. Veraldi says. “It will step up in heme, it will step up in blood, it will become darker red with serial lavage if it’s truly diffuse alveolar hemorrhage.” Alternatively, she says, if the returned lavage clears with each aliquot, the bleeding is not consistent with DAH. It’s imperfect, she adds, but it is a good diagnostic clue about whether what is seen is truly diffuse alveolar hemorrhage.
 Dr. Schneider says it can be hard to recognize capillaritis with certainty (Fig. 16). “If you have a patient with diffuse alveolar hemorrhage, it’s helpful to be extremely alert and to look for it and maybe lower the threshold of what might be or what might highlight a capillaritis, depending on the size of the sample.”
Dr. Schneider says it can be hard to recognize capillaritis with certainty (Fig. 16). “If you have a patient with diffuse alveolar hemorrhage, it’s helpful to be extremely alert and to look for it and maybe lower the threshold of what might be or what might highlight a capillaritis, depending on the size of the sample.”
In the wedge biopsy, there is more to look at and thus a better predictive value of what is seen can be offered, he says, but on a transbronchial biopsy, it may have been missed because it can be patchy.
In Fig. 17 there is diffuse alveolar hemorrhage—fibrin in the air space, red blood cells, but no hemosiderin-laden macrophages (“You just have to believe that this is a patient with diffuse alveolar hemorrhage who does have capillaritis,” Dr. Schneider says). There is a very small vessel, even smaller capillaries, going through the alveolar septa, and there are neutrophils surrounding it.
“They often expand the septum a little, they tuft little clusters, and then they break out. They destroy the alveolar septum and they’re caught up in the adjacent alveoli with some fibrin,” Dr. Schneider says.
Fig. 18 looks like an acute and organizing lung injury with pneumocyte hyperplasia and slightly edematous alveolar septa. It has a bit of fibrin, red cells, a few hemosiderin-laden macrophages. “If you look at the interstitium, very subtle and very few neutrophils,” he says. “For me, it would be essentially impossible to make a diagnosis on this specific field.” In Fig. 19 there is an unusual clustering of neutrophils. “You might find some fragments of cells, some extravasated red cells in the interstitium, all signs in the proper background of diffuse alveolar hemorrhage that could highlight capillaritis.”
What you don’t want to see in capillaritis is more neutrophils present in the air space than in the actual interstitium. If there are more neutrophils in the air space with fibrin than in the interstitium, “that’s usually a good sign that you’re looking at primary air space disease and that is usually a pyogenic infection,” Dr. Schneider says.
 What advice should be given to a clinician regarding whether immunofluorescence studies should be done or if fresh tissue should be submitted? Dr. Schneider asks.
What advice should be given to a clinician regarding whether immunofluorescence studies should be done or if fresh tissue should be submitted? Dr. Schneider asks.
Make sure the pulmonologists and surgeons are on the same page, Dr. Veraldi replies, and get them into the habit of submitting tissue for immunofluorescence every time. “It’s better than being surprised by findings that require immunofluorescence for further evaluation,” she says, “because no one wants to go back for a second surgical biopsy or bronchoscopy if it can be avoided by good advance planning. There’s no harm in an extra snippet of tissue to have in reserve to be able to do these studies if they’re necessary. So train your pulmonologists and surgeons.”
From a pathology perspective, the harm in not looking at a piece of tissue is that “we could be missing information,” Dr. Schneider says. At what point does the pathologist need to look at that piece on an H&E slide rather than do immunofluorescence on it? “I’m not sure there’s a good answer for that,” he says. Dr. Veraldi replies: “The whole clinical care team can help with the decision about what is the most important question to ask with the remaining tissue we have.”
Dr. Schneider recapped the main points:
- The inflammation of small vessels in the lung, yet not capillaries, is relatively nonspecific, often a secondary phenomenon. “You need good clinical correlation, mention it in the report, but no one is going to go run with it.”
- The hallmark feature of the smallest vessel vasculitis, capillaritis, is diffuse alveolar hemorrhage. So capillaritis is, of all the 2012 Chapel Hill classification vasculitides, the one that allows a category to be assigned most confidently. “It’s still a broad differential diagnosis, but it boxes you in on what the differential diagnostic considerations could be. So whenever you see capillaritis, you want to mention it. Whenever you see diffuse alveolar hemorrhage, you want to look carefully for capillaritis. And usually it’s fine if the pathologist diagnoses a specimen as ‘Lung, left lower lobe, wedge biopsy: diffuse alveolar hemorrhage, with capillaritis.’”
Sherrie Rice is editor of CAP TODAY.