Karen Lusky
July 2022—Gut pathogens, their histologic features, and a GI pathology and microbiology team approach to diagnosis were the focus of a CAP21 session, “What’s Bugging the Gut?”
Maryam Zenali, MD, Alina Iuga, MD, and Christina Wojewoda, MD, presented a series of cases and highlighted the features, the differential diagnoses, and the integrated workups. Three of their cases follow here, with others to be reported in an upcoming issue.
The patient in the first case is a 14-year-old boy who presented with fever, bloody diarrhea, and abdominal pain. Endoscopic evaluation revealed mucosal edema of the right colon extending to the ileocecal valve and scattered ileocecal ulcers. Due to the severity of symptomatology, a laparotomy was performed shortly thereafter.
The resection specimen contained suppurative granulomatous appendicitis and active chronic enterocolitis (Figs. 1–3). Granulomatous inflammation was also noted in the endoscopic biopsies of the right colon (Fig. 4), explained Dr. Zenali, assistant professor of pathology, Department of Pathology and Laboratory Medicine, UConn Health.
“The differential diagnoses include infectious disease such as Yersinia, salmonella, or mycobacteria enterocolitis and Crohn’s disease,” she said.
This is where the microbiology laboratory can be of help, said Dr. Wojewoda, director of microbiology at the University of Vermont Medical Center and associate professor, University of Vermont Larner College of Medicine. If the patient had submitted a stool culture before returning for further surgery, she said, they could have diagnosed Yersinia enterocolitica. “Ideally we would incubate the stool at 25 degrees [Celsius] because Yersinia likes to grow at cooler temperatures.” Not every laboratory will automatically culture for Yersinia enterocolitica, “so this is where the pathologist can interact with the GI clinician or surgeon to ensure we know what we are supposed to be looking for.”
If they were to culture for Yersinia, Dr. Wojewoda said, they would look for lactose-negative colonies on the MacConkey agar. “There is a selective and differential agar for Yersinia enterocolitica called CIN agar,” on which red bull’s-eye colonies form. It can take about 48 hours to grow, and then two to 24 hours to identify the organism. PCR studies on stool are available for Yersinia enterocolitica, “although these are usually incorporated into large multiplex panels, which usually are overkill,” she said, “unless you are looking for something rare.”
Yersinia enterocolitica is a Gram-negative rod in the order Enterobacterales (previously Enterobacteriaceae). Yersinia enterocolitica and Yersinia pseudotuberculosis can be found in meats (most commonly pork), dairy products, and water. The CDC estimates it causes almost 117,000 illnesses, 640 hospitalizations, and 35 deaths in the U.S. yearly, with children infected more often than adults.

Fig. 1. Appendix H&E 40×
Yersiniosis often involves the terminal ileum/ileocecal region and mesenteric lymph nodes; it may also affect the appendix. Suppurative and granulomatous patterns are common in Yersinia enterocolitica and in particular in Yersinia pseudotuberculosis, Dr. Zenali said. “Patients can have concurrent mesenteric lymphadenitis. The organism accesses the bowel wall through the microfold [M] cells of the Peyer’s patches, so the inflammatory reaction often initiates from the terminal ileum and ileocecal valve and can further spread to the right colon.”
In immunocompromised patients Yersinia is a severe disease, as it is in patients with iron overload. “We don’t know the exact mechanism for that,” Dr. Zenali said of overload, but “we know the organism needs iron for growth, and its growth is hindered when iron is scant.”
The histology, although suggestive, is not entirely specific for Yersinia infection, and culture and/or PCR are the gold standard for definitive diagnosis.
“Mycobacteria would have caseating granulomas, and you can identify acid-fast bacterium in some of the cases, if not all,” Dr. Zenali said. Mycobacteria also favor the ileocecal valve region, as does salmonella. With salmonella, small, loose to well-formed granulomas, with neutrophilic microabscess, can be seen.
Similar to inflammatory bowel disease/Crohn’s disease, chronic bacterial infection can lead to mucosal architectural distortion. With Crohn’s disease, confluent granulomas are less likely and perianal disease or fistula more common.
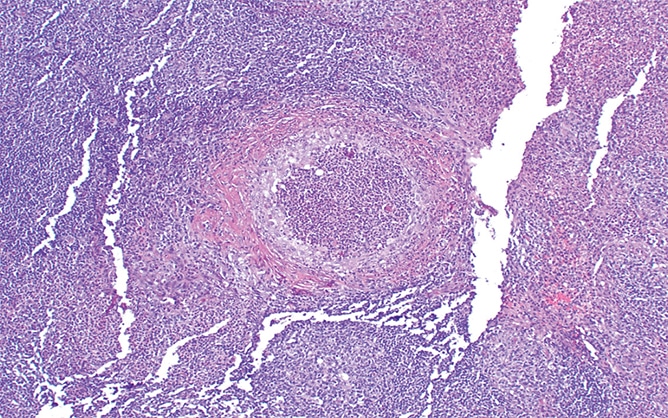
Fig. 2. Appendix H&E 100×
Most cases of Y. enterocolitica and Y. pseudotuberculosis are self-limited. If treatment is needed, supportive care might be sufficient. Antibiotics are prescribed for those with more severe disease or intractable symptoms. Severe disease may lead to intestinal perforation.
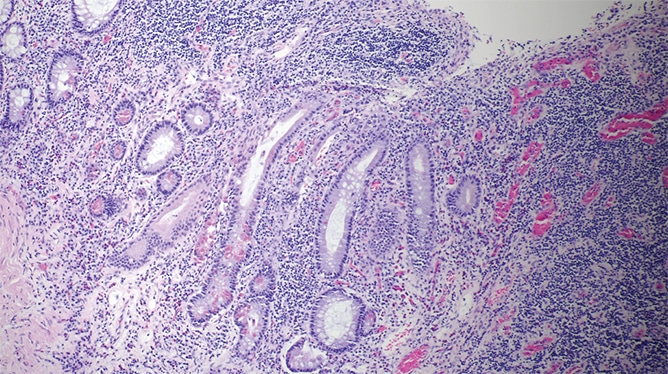
Fig. 3. Resected bowel H&E 100×
Studies report identification of Yersinia by PCR in the normal colon of asymptomatic patients and also in patients with Crohn’s disease. “So the question is what is the bacterial load,” Dr. Zenali said. “We also have to interpret the results in the context of the clinical presentation.”
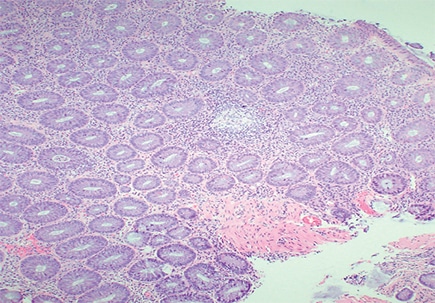
Fig. 4. Right colon biopsy H&E 40×
The second case is that of a 35-year-old woman with abdominal pain referred for celiac disease evaluation based on initial histologic findings of the upper gastrointestinal endoscopic samples. The duodenal biopsy showing mild villous blunting is seen in Fig. 5. At higher magnification (Fig. 6) mild intraepithelial lymphocytosis and mild lamina propria expansion by lymphoplasmacytic infiltrate are seen.
Seen in Fig. 7 is active inflammation consisting of neutrophils in lamina propria and focally in the epithelium. “Active inflammation may be seen in celiac disease as a marker of active disease, in particular in pediatric cases,” said Dr. Iuga, director of gastrointestinal pathology and associate professor, Department of Pathology and Laboratory Medicine, University of North Carolina School of Medicine. “However, when we see it, we should keep in mind the differential diagnosis that includes peptic injury, a drug effect such as NSAIDs, infectious etiology, and even inflammatory bowel disease.”
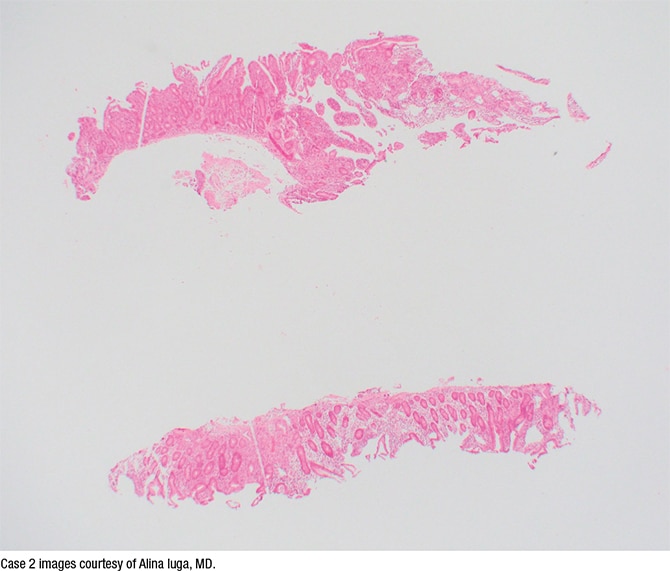
Fig. 5. H&E stain, 2×
In Fig. 8 small basophilic bodies are seen on the luminal surface of the enterocytes, and thus the differential diagnosis includes infectious parasitic organisms such as Cyclospora cayetanensis, Cystoisospora (Isospora) belli, Cryptosporidium, and Microsporidium. “The first three are members of Coccidia, which is a subclass of microscopic spore-forming, single-celled, obligate intracellular parasites that can infect epithelial cells lining the digestive tract of humans and animals,” Dr. Iuga explained. On histology, they are usually distinguished by size and location. Cryptosporidium (4–6 µm) is usually located at the luminal surface of the enterocytes. Cystoisospora is larger, averaging 20 to 30 µm in size, and Cyclospora measures 8 to 10 µm. They are usually observed inside the enterocytes. “Microsporidia, which average 1.5 to 2 µm, were formerly considered spore-forming protozoa and have been reclassified as intracellular fungi.”
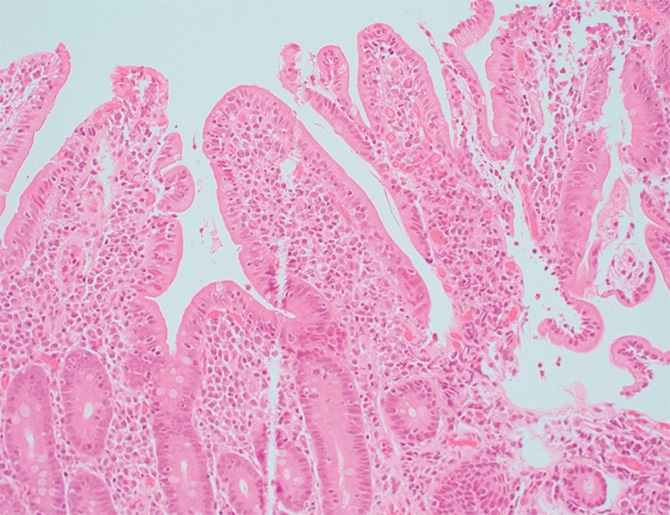
Fig. 6. H&E stain, 20×
In Fig. 9 is a Giemsa stain highlighting the organisms. “Based on the morphologic appearance and the location at the surface of the enterocytes, these are compatible with Cryptosporidium,” Dr. Iuga said.
Here, too, the clinical laboratory can be of help, Dr. Wojewoda added. “If this patient had submitted a stool sample, immunofluorescent microscopy could have been performed. We can do an antigen assay for Cryptosporidium or PCR.”
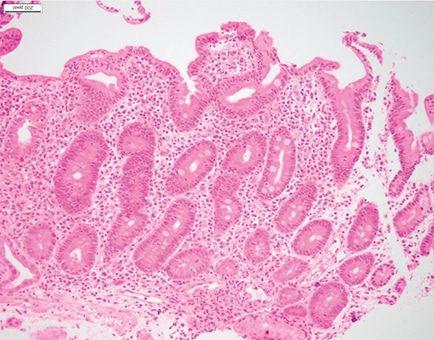
Fig. 7. H&E stain, 20×
Cryptosporidium is not detected well in a routine ova and parasite exam, “which is something we don’t teach medical students very well,” Dr. Wojewoda said, adding, “I’m working on it.” In a routine O&P exam, Cryptosporidium can look like yeast, which are normal in stool. “This is a time when you can work with your GI, family medicine, and infectious diseases colleagues. Make sure they know that either a Cryptosporidium exam, antigen assay, or PCR assay is a better test.”
If they do an O&P exam, a modified acid-fast stain would be used to differentiate Cryptosporidium from yeast. “When we observe them, the oocysts are approximately four to six microns and very round.”
Multiple Cryptosporidium species can cause disease, she said, but Cryptosporidium parvum and Cryptosporidium hominis are the most common. “We get this via ingestion of unclean food or water that’s been contaminated with stool,” the latter often encountered in water parks. In 2018 there were 748,000 cryptosporidiosis cases.
Cryptosporidium organisms show small basophilic round bodies protruding from the apex of enterocytes, highlighted with Giemsa stain. “Interestingly, they are considered intracellular but extracytoplasmic in location,” Dr. Iuga said. They are located within parasitophorous vacuoles covered by the host’s microvillous membranes. “Histologically,” she said, “the small intestine mucosa can show variable villous atrophy, variable inflammation including cryptitis and lamina propria neutrophilic infiltrate, as well as intraepithelial lymphocytosis and eosinophil infiltrate.”

Fig. 8. H&E stain, 60×
Cryptosporidium can be found in lakes and rivers, especially when the water is contaminated with sewage and animal waste, and it’s resistant to chlorine disinfection and therefore can be present in drinking water. The disease is caused by ingesting infectious oocytes followed by the release of sporozoites that invade enterocytes, primarily in the small intestine. The most common sites of infection are the small intestine and proximal colon, “but they can also infect the distal colon, pancreas, respiratory tract, and, interestingly, the gallbladder and biliary tree. And there are reported cases of sclerosing cholangitis associated with Cryptosporidium infection,” Dr. Iuga said.
Cryptosporidium can cause self-limited diarrhea in immunocompetent patients and chronic diarrhea in immunocompromised patients. “It can also be a cause of malabsorption, lactose intolerance, dehydration, weight loss, and malnutrition in severe cases,” she noted. Infection is managed with supportive therapy, antiprotozoal medication, or antiretroviral therapy in HIV patients or by reducing immunosuppression in other settings.
The third case is that of a 42-year-old man who presented with lower abdominal pain and hematochezia. On physical examination there were a few slightly raised, painful perianal tags. Colonoscopy revealed erythema and discrete ulcers in the rectum. Abdominal CT scan revealed circumferential thickening of the rectal wall and numerous enlarged perirectal lymph nodes, concerning for malignancy.
H&E sections from the perianal tags (Figs. 10 and 11) showed psoriasiform epidermal hyperplasia with superficial and deep perivascular and periadnexal chronic inflammatory infiltrate, Dr. Zenali explained.
Chronic inflammatory infiltrate is often lymphoplasmacytic with scattered histiocytes and eosinophils. Another histologic finding in syphilis is obliterative endarteritis, characterized by concentric endothelial swelling, fibroblastic thickening, and narrowing of the vascular lumen. Fig. 12 is a photomicrograph of Treponema pallidum immunostain highlighting the presence of spirochetes in the epithelium.
Microscopic examination raises a diagnosis of condyloma lata, which is distinct from the more commonly encountered anogenital condyloma (condyloma acuminatum). Condyloma lata occur in the setting of secondary syphilis. “On H&E alone,” Dr. Zenali said, “we cannot entirely exclude other venereal disease such as lymphogranuloma venereum [LGV] or granuloma inguinale [donovanosis], and immunostaining or silver impregnation methods can aid in further delineation.”
A biopsy was needed, Dr. Wojewoda said, in addition to a syphilis serology workup for confirmation. The nontreponemal serology (Venereal Disease Research Laboratory, rapid plasma reagin) used to be performed first, “and if that was positive, you would confirm with treponemal-specific serology,” such as the fluorescent treponemal antibody absorbed (FTA-ABS) test or T. pallidum particle agglutination (TPPA).
The treponemal serology is now automated and it’s therefore easier to do the treponemal serology as the first line. “If that serology is positive, we reflex to a nontreponemal serology, such as the RPR.” If tissue is available, Treponema pallidum would be positive by Warthin-Starry stain, and there’s also an organism-specific immunohistochemical stain that can be performed on tissue,” Dr. Wojewoda said.
Almost 130,000 cases of syphilis were diagnosed in 2019, a 74 percent increase since 2015. “And the scary part,” she said, “is that we saw a 279 percent increase since 2015 in congenital syphilis cases,” with 1,870 cases diagnosed in 2019, “and that trend is continuing today.”
In the digestive tract, Dr. Zenali said, syphilitic disease more frequently manifests in the anorectal region, but it can also affect other sites. “Case studies of syphilitic hepatitis and severe gastritis have been reported. Due to its variable presentations, syphilis is known as the great imitator. Thus, underdiagnosis or misdiagnosis rates can be high,” she said.
In primary syphilis, the lesions generally appear at the site of first inoculation, and so-called chancre(s) can be single or multiple. In secondary syphilis, there is rash and adenopathy, and condyloma lata is the characteristic lesion, often occurring about six to eight weeks after the primary infection. Features of primary and secondary syphilis can coexist. Patients with syphilis may even remain asymptomatic for long periods. Neurological disorders, arthritis, and a distinctive lesion called gumma are seen in tertiary syphilis, she explained.

Fig. 9. Giemsa stain, 60×
On histology, in nearly any stage of syphilis, there is increased chronic inflammation and obliterative endarteritis, Dr. Zenali said. “In the luminal GI tract, secondary syphilis can manifest as ulcers, cryptitis, mass-like chronic inflammation, or even granulomatous inflammation.” The spirochete organism can be identified in primary and secondary lesions, “but it can be difficult to demonstrate in the gummas.”
“We always want to see a lot of plasma cells in syphilis, but occasionally they are few if any,” Dr. Zenali said, adding, “Sometimes you just see a lot of eosinophils and lymphocytes or histiocytes.” It’s something to keep in mind, she said: “If you don’t have a lot of plasma cells, that doesn’t rule out syphilis histologically. If there is any suspicion, get the appropriate stains.”

Fig. 10
Of the differential, Dr. Zenali said condyloma acuminatum is typically a firm, raised, keratinized anogenital lesion, and HPV-associated cytopathic effect can be seen in many, if not all, cases.
Like syphilis, Chlamydia trachomatis can cause coloproctitis. At the inoculation site it often forms papules and ulcers, which quickly disappear and become fibrotic; it may become a stricturing disease or develop fistulae. “You may find enlarged inguinal lymph nodes with stellate abscess inside of them.”

Fig. 11
The perianal disease in donovanosis or granuloma inguinale (caused by Klebsiella granulomatis) forms nodular or ulcerated lesions. The condition can clinically mimic syphilis and lymphogranuloma venereum, she cautions. In granuloma inguinale, lesions contain pathognomonic inclusions called Donovan bodies within the mononuclear cells. “They are distinctive in that they are bipolar-staining intracellular densities on standard Giemsa or silver stains.”

Fig. 12. Treponema pallidum IHC 40×
Penicillin is the treatment of choice for syphilis, Dr. Zenali said, but the regimen is stage dependent. The RPR test can be used to monitor treatment, she said, and it is important to be familiar with the caveats of post-treatment RPR levels to avoid misinterpretation. “For treatment purposes, knowing the patient’s HIV status is important and typically assessed due to risk of co-infection.”
Karen Lusky is a writer in Brentwood, Tenn.