Valerie Neff Newitt
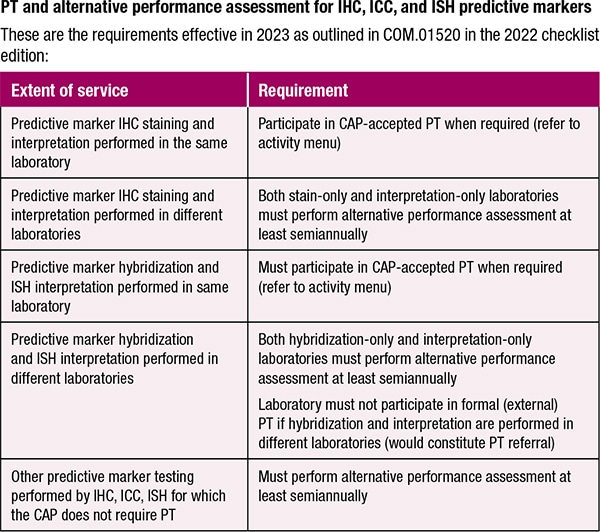
September 2022—Beginning next year, two additional predictive marker tests will require enrollment in proficiency testing (PT), but for any predictive marker using immunohistochemistry or in situ hybridization, only laboratories that perform both staining and interpretation must participate in CAP-accepted PT.
For laboratories that perform only the staining or only the interpretation, but not both on site, alternative performance assessment will be required at least semiannually. Previously, staining-only laboratories were not required to perform proficiency testing or alternative assessment. For interpretation-only laboratories, it is a move away from proficiency testing to alternative performance assessment.
These requirements overall reflect “an urgent need to assess the quality of predictive marker testing because of the incredible importance of the decisions being made on these predictive markers leading to targeted therapies,” says Bradley S. Karon, MD, PhD, chair of the CAP Council on Scientific Affairs and professor of laboratory medicine and pathology at Mayo Clinic in Rochester, Minn. “But we’re doing it in a way that is going to allow us to assess the largest sources of variability or differences in quality without placing enormous burden on practices and labs to enroll in redundant Surveys.”
Avoiding that burden will come with the CAP’s shift from an interpretation-centric model for predictive marker testing to a staining-centric model, he says, “where the requirement for proficiency testing eventually falls mainly to the lab doing the staining and interpretation.” In a typical hub-and-spoke model where slides are stained and interpreted in a central (hub) laboratory, but this lab also sends stained slides to other labs (spokes) for interpretation, proficiency testing will now be required only for the hub laboratory, Dr. Karon says.
Under the new plan, the spoke labs that are performing interpretation only will not be required to enroll in a formal proficiency test, “because that model became burdensome and expensive” and couldn’t be expanded to accommodate new predictive markers, Dr. Karon says. Proficiency testing is still an option, but these laboratories can choose to perform another form of quality assurance, and the CAP is exploring new educational programs to meet the need. The programs would enable laboratories to assess not only the ability of pathologists to interpret but also the consistency of the interpretation among pathologists in a group or at a site.
The two predictive markers for which enrollment in proficiency testing will be required for the first time in 2023 are HER2 IHC in gastroesophageal adenocarcinoma and highly sensitive (hs) ALK in non-small cell lung cancer. The other two for which proficiency testing has been required for years are estrogen receptor and HER2 in breast cancer. “Other analytes deserve the same scrutiny as the breast biomarkers,” explains Andrew M. Bellizzi, MD, chair of the CAP Immunohistochemistry Committee and clinical professor in the Department of Pathology, University of Iowa Hospitals and Clinics.
The difficulty of IHC tests can vary, he notes. “Some are much more challenging, more finicky, in need of more attention.” Gastric HER2 and hsALK are two such tests, he says. “Because they are hard and the results are so critical to therapeutic decision-making, they demand more attention than the vast majority of analytes we look at by immunohistochemistry.” Of all the non-breast predictive markers, Dr. Bellizzi says, “these are the most problematic.”
The CAP’s plan for the oversight is found in the 2022 edition of the accreditation program checklists to be released next month, in COM.01520 PT and Alternative Performance Assessment for IHC, ICC, and ISH Predictive Markers. There it says the laboratory that performs IHC staining and interpretation must participate in CAP-accepted PT when it’s required, per the lab’s activity menu, and the stain-only and interpretation-only labs must perform alternative assessment at least semiannually.
It also says the laboratory that performs both predictive marker hybridization and ISH interpretation on site must participate in CAP-accepted proficiency testing. Labs that perform only one of the services must perform alternative assessment. These hybridization-only and ISH interpretation-only labs are not permitted to enroll in a formal proficiency testing program because, as the requirement notes, “participation in formal PT would constitute PT referral.”

Dr. Karon
For all other predictive marker testing performed by IHC, ISH, and immunocytochemistry for which the CAP doesn’t require proficiency testing, alternative performance assessment at least semiannually is required.
Dr. Bellizzi calls the lab’s activity menu “the most important piece in allowing us to move forward” with the PT requirement for gastric HER2 and hsALK.
The CAP has revised the activity menu so that it is aligned with the new proficiency testing and alternative performance assessment requirements for predictive marker testing using IHC. It now differentiates activities for each predictive marker to indicate clearly the staining and/or interpretation services the laboratory performs on site.
Not paying sufficiently close attention to the activity menu is a problem for some labs, says CAP checklist editor Lyn Wielgos, and an incomplete or incorrect activity menu is a common deficiency found when laboratories are inspected. “We also identify activity menu issues outside of the on-site inspection during follow-up after inspections or when working with laboratories on proficiency testing failures. Making sure the activity menu is correct is a key part in making sure this all makes sense and works,” she says of the requirements for predictive marker tests.
Says Dr. Bellizzi, “If labs are not telling us exactly what they are doing, then we’re limited in our ability to oversee.” Thus, the CAP is planning to offer education targeted to the updates to the activity menu and to the new proficiency testing requirements for predictive markers.
His advice: “Take a look at your activity menu, understand these three different ways to interface with the biomarker, understand what it means to do only staining, to do only interpretation, what it means to do staining and interpretation. If you don’t understand, ask for help and confirm that your activity menu is correct. If not, update it to make it as correct as possible.”
The education geared toward the activity menu will be aimed at eliminating confusion. “I think there is quite a bit of confusion now,” Dr. Karon says. “So we hope we’re going to clear up confusion about what labs should be doing about the PT and alternative performance assessment.”
“A lot of this is about education,” Dr. Bellizzi says of the activity menu and the educational program under study for interpretation-only laboratories. “We don’t want labs that are struggling with this class of testing to necessarily stop doing the testing. First we want to highlight that they’re doing this testing suboptimally, and then if it’s important to them to continue with the testing, we want to provide them resources to improve their testing.”
The needs of laboratories that stain and interpret differ from those for labs that only interpret, “and the next big push” will be to create products that meet the needs of the latter, he says. “Our preference is to create products so labs don’t have to think so hard about how to design alternative assessment.”
Knowing what PT or alternative performance assessment is required and following through is one thing. Addressing a failure is another, and failing to address a PT failure is important to avoiding a citation for a deficiency, says Harris S. Goodman, MD, chair of the CAP Checklists Committee, member of the CAP Council on Accreditation, and chief of the Department of Pathology, Alameda Health System Highland Hospital, Oakland, Calif. “One of the things I most commonly cite when I do inspections is when there’s been a PT failure and no one did anything about it,” he says. A laboratory can perform satisfactorily on a proficiency test event—get nine out of 10 challenges correct—but all failures have to be investigated.
Laboratories need a procedure for addressing such failures, and most labs do, Dr. Goodman says. “It’s just that they don’t actually do it. So I come across a PT failure and I say, ‘How did you address this?’ And they say, ‘Well, it was only one out of 10, so we’re still in compliance.’ And I say, ‘But you still have to address the failure.’”
An investigation report is common in laboratories, he says, one that looks at the result obtained and the result expected, among other things. In his own laboratories, a checklist is used. “Was it a transcription error? Was the specimen not reconstituted properly? Not run properly? There’s a whole list of things we go through to see why it failed. And most labs have a procedure that says to do that. They just don’t necessarily do it.”
Dr. Goodman cautions, too, about inadvertent proficiency testing referral for laboratories that perform in situ hybridization. If the ISH and interpretation are performed in different laboratories, participation in formal PT would constitute PT referral. “Even if that’s the way patient specimens are handled, they are going to have to do an alternative performance assessment instead for ISH,” he says.
For predictive markers, he says, the requirements are method specific. He cites HER2 as an example. “If labs do different methods—IHC or FISH—to assess HER2 status, they have to participate in PT for both methods. They can’t just do one.”
For pathologists, predictive marker testing differs from other types of testing in which multiple characteristics are used to determine choice of therapy, Dr. Goodman notes. “With all these predictive markers, it’s just a single test, and so much rides on the single test, making it extremely important that we’re doing the test well.” Behind the CAP’s decision is its expectation that a lab perform its predictive marker testing well and use proficiency testing or alternative performance assessment to ensure it is performing well, and address problems if it’s not, he says.

Dr. Bellizzi
The CAP will offer webinars to guide labs in how to comply with what will be required, as well as a CAP22 session, “Tips and Tools for Quality Planning, Predictive Marker Monitoring, and Process Improvement in IHC,” led by Emily Meserve, MD, MPH, Russell Higgins, MD, and Dr. Bellizzi. In the 90-minute session to take place Oct. 9 on site, they will discuss the elements of an IHC laboratory quality plan, explain the requirements for the monitored IHC predictive markers, present case-based studies for process improvement of selected markers, and talk about strategies to be applied to additional markers, such as PD-L1, in the future.
The requirements for proficiency testing and alternative assessment for predictive markers are a way to “close the gaps so there’s a structure to hold accountable the labs that are doing this type of testing,” Dr. Bellizzi says. And the upcoming planned instruction will provide the assistance that some laboratories will need.
Limiting redundant Surveys enrollment is one benefit labs can expect to see, Dr. Karon says. “We’ll be reducing redundant proficiency testing for labs that might have had to enroll in five or six separate Surveys and that now may enroll in one per predictive marker.” And that is the goal as the number of markers requiring proficiency testing grows, he says: “to make a model that is not so burdensome for labs that they just can’t afford to do this anymore.”
Valerie Neff Newitt is a writer in Audubon, Pa. For a list of frequently asked questions from immunohistochemistry laboratories, and answers to those questions, go to https://bit.ly/CAP-IHC-FAQ.