Charna Albert
May 2023—Of the five methods used to measure HbA1c—immunoassay, boronate affinity, enzymatic, capillary electrophoresis, and ion-exchange HPLC—only the latter two can alert the laboratory and physician to the presence of a suspected hemoglobin variant. Even so, there are reasons to be cautious.
Immunoassay is the predominant method used today, and by immunoassay, “the hemoglobin variants are not well detected if at all,” said David Koch, PhD, D(ABCC), professor of pathology at Emory University, in an AACC session last year on HbA1c results in patients with hemoglobin variants. He and Jeanne Rhea-McManus, PhD, MBA, D(ABCC), NRCC, senior director of medical science information and communication at Siemens Healthineers, and joined by Lucia Berte, MA, MT(ASCP)SBB, DLM, principal, Laboratories Made Better, Broomfield, Colo., highlighted the HbA1c methods and variants and their effect on patient results, and they put forth an algorithm that takes all of it into account.
Diagnostic companies have been able to eliminate analytical interference from the common variants, Dr. Rhea-McManus said, “but there are hundreds more variants” that may interfere. “And that can have different ramifications.”
If a patient’s HbA1c value differs from clinical expectations, that’s an indication that a hemoglobinopathy interference with measurement or interpretation could be occurring, “especially if the method you’re using isn’t able to identify the presence of a presumptive variant,” she said. And if the HbA1c value is greater than 15 percent, “or even if it’s below the analytical measurement range,” that also suggests additional investigation should be carried out. If blood glucose and HbA1c values are discordant—“if a patient comes in and their blood glucose logs show they’re not in good glycemic control, but their hemoglobin is 5.1 percent,” for example—further investigation is needed. And if there’s a dramatic change in the HbA1c value from a prior reading following a change in methods, that also suggests additional investigation is warranted.
HbA1c is typically reported as a percent of total hemoglobin, said Dr. Koch, who is also director of clinical chemistry, toxicology, and point-of-care testing at Grady Memorial Hospital in Atlanta. The amount of hemoglobin A1c relates to the concentration of glucose in the circulation, “but what is often ignored or forgotten or not understood is that it’s also related to the lifespan of the red blood cell. And if that is altered in any way, that’s going to alter what we measure in terms of hemoglobin A1c.”

Dr. Koch
In a hematologically normal person, average red blood cell lifespan is about 120 days. “So then the hemoglobin A1c that we measure in the laboratory reflects the integration of glucose over the preceding eight to 12 weeks,” he said.
Nearly all the HbA1c methods rely on one of two features of HbA1c that allow it to be distinguished from the other hemoglobins, he said. Ion-exchange chromatography and electrophoresis use the charge differences, and boronate-affinity chromatography and immunoassay use the structural differences, between HbA1c and the other hemoglobins.
Cation-exchange high-performance liquid chromatography (HPLC), which was used in the Diabetes Control and Complications Trial, is the second most common method in use today. It works by applying buffers of increasing ionic strength to the cation-exchange column, which then elutes the different Hb species at separate times. “The concentration is measured after elution from the column and that’s used to quantify the percent of hemoglobin A1c by calculating the area under each peak from the ion-exchange chromatography,” Dr. Koch explained. “And variant hemoglobins will affect the results in a variety of ways.” Variants that elute separately from HbA or HbA1c will have little or no effect on HbA1c values. “Unfortunately, we have some variants that will elute with the hemoglobin A1c peak, and that will cause a falsely high hemoglobin A1c result. Or we could have a variant that elutes with the hemoglobin A peak, and that’s going to cause a falsely low hemoglobin A1c value.”
With ion-exchange chromatography, “we often will see the presence of these variants,” he said. “So we’re not going to be dependent on the [false] A1c result, but we’ve got to pay attention to what the chromatograph is telling us.”
Electrophoresis was the original method used to measure HbA1c. “Better methods were developed over the years so electrophoresis faded away—it was hardly ever used,” Dr. Koch said. “But capillary electrophoresis is a recent development and several methods are now available commercially. So it’s coming back into use, though it’s still about the fourth most common technique. And variant hemoglobins usually are seen by capillary electrophoresis and therefore do not cause analytical errors.”
In boronate-affinity chromatography (third most common), the boronate columns retain glycohemoglobin while “all the other hemoglobins elute early,” and then HbA1c is derived from the two peaks. “The variant hemoglobins affect the analytical value very little, if at all, but the presence of variant hemoglobins is not detected. So you don’t know if you have one and if you might therefore have an altered red blood cell lifespan.” Several commercial methods are available.
Immunoassay works by raising antibodies against the glucose linkage with the first four to eight amino acids on the N-terminal of the beta-chain of hemoglobin. “The signal is usually a light scattering or turbidimetric signal that’s caused by agglutination of the antibody and the molecule you’re trying to measure.”
The turbidimetric immunoassays typically contain latex particles with attached antibodies for the HbA1c structure. But a synthetic glycated peptide also is part of the reagent system, he said. “And then we have a detector and what we do is add sample, incubate, and then measure the signal.” If little or no HbA1c is in the sample, the antibodies will be attracted to the synthetic glycated peptide, causing agglutination. “And then the signal to the detector is reduced, which really means the turbidimetric signal is increased—it’s high, because of that situation.” If HbA1c is present, the HbA1c molecules will bind to the antibodies, rather than the synthetic glycated peptide. This increases the signal to the detector, he said, “because there’s not much agglutination. And that means the immunoturbidimetry signal is reduced. So we have a signal that’s related to the output, and we can then measure the hemoglobin A1c.”

Dr. Rhea-McManus
An enzymatic technique that uses fructosyl peptide oxidase has become available in recent years. The fructosyl peptide oxidase enzyme cleaves the fructosyl dipeptide from the N-terminal of the beta-chain of HbA1c, hydrolyzing the dipeptide and producing a chromogen, which can be measured. The signal it emits is proportional to the amount of HbA1c in the sample. Several commercial assays are on the market, Dr. Koch said, but use is likely low. “We still don’t know what the hemoglobin variants are going to do to these methods, so we need to be careful about that.”
In patients with clinical conditions that affect red blood cell lifespan, Dr. Rhea-McManus said, HbA1c results, too, may be affected. In iron deficiency anemia and folate or B12 anemias, for instance, RBC lifespan is increased, and that can cause a falsely elevated HbA1c result. “Or if you have clinical scenarios that lead to decreased red blood cell survival”—chronic blood loss, hemolytic anemia, or pregnancy, for example—“that can lead to falsely decreased levels of hemoglobin A1c.” And elevated levels of fetal hemoglobin, which usually makes up a small fraction of total adult hemoglobin, also can lead to decreased HbA1c.
HbS, HbC, HbE, and HbD are the predominant hemoglobinopathies worldwide. The analytical interferences these variants cause have been well characterized for most HbA1c methods, she said. “And manufacturers are doing a great job of developing methods that aren’t susceptible to analytical interferences from these variants.” The NGSP, she noted, publishes a list of HbA1c assay interferences (https://shorturl.at/iCEN5).
HbA1c will accurately reflect a patient’s glycemic state only if RBC survival is constant over the 120-day lifespan, Dr. Rhea-McManus said. In addition, RBCs must be freely permeable to glucose, “so that nonenzymatic glycation of the hemoglobin molecule can occur.” And hemoglobin A glycation must occur at a rate directly proportional to ambient glucose concentration. If any one of these conditions doesn’t hold true, HbA1c concentration may no longer represent the average blood glucose value. “And it may not accurately reflect what’s happening with the patient.”
The predominant hemoglobin variants more commonly present in the homozygous form, meaning the patient lacks hemoglobin A. “This oftentimes impacts red blood cell lifespan,” Dr. Rhea-McManus said. When RBC survival is decreased, the cells have less time to be exposed to the glucose in circulation. “And if you compare it to a normal red blood cell, you’ll see a decreased A1c.” If survival is increased, “you’re likely to see a falsely elevated A1c value, because that red cell has been hanging out and has had more time to be glycated through that nonenzymatic process.”
In a healthy adult, hemoglobin A makes up about 90 percent of total hemoglobin. HbA1c makes up three to nine percent of the total, HbF less than two percent, and HbA2 two to five percent. “And when you have mutations in the hemoglobin chain, either in the alpha-chain or beta-chain, that gives rise to the hemoglobin variants,” she said, which can be grouped largely into structural or quantitative defects. To date, more than 1,200 structural variants have been identified, thought to be due mainly to the selective advantage that occurs in the presence of malaria. The structural defects can arise from different mutation types, such as a point mutation or an insertion or deletion in the sequence, but the globin chains still are produced. “They’re incorporated into the total hemoglobin molecule, and that can have downstream consequences.”
The quantitative defects affect the amount of alpha- or beta-chain produced. “That can lead to just a small reduction in one of those two chains, or it can lead to a complete absence of one of the globin chains,” Dr. Rhea-McManus said. “The thalassemia syndromes fall into this bucket.” And it’s possible to have both structural and quantitative defects in hemoglobin, known as a compound heterozygous variant. “For example, a hemoglobin S/beta thalassemia patient has a structural defect with their hemoglobin S, and they also have a quantitative defect with beta thalassemia.”
Average RBC lifespan, she said, is decreased in patients with the common Hb variants. In those with sickle cell trait (HbAS), for example, average RBC lifespan is 93 days, and it’s 87 days in those with HbC trait. RBC lifespan in those with HbD trait is closer to normal, at 115 days. And for HbE, “there’s not enough data to know if there is an impact on red blood cell lifespan and if so what that magnitude is,” she said.
Though assay manufacturers have been successful at eliminating HbA1c analytical interferences from the most common variants, there are hundreds of other variants, she said. “It’s possible that if you’re using a method where you can’t see a hemoglobin variant, it may interfere with that assay.” There are also mutations at glycation sites. “The glycation rate needs to be directly proportional to the amount of glucose present.” So a mutation that affects how quickly or slowly the molecule is glycated also can change the relationship between HbA1c and the glucose values it’s supposed to represent, she said.
In patients with the homozygous forms of HbS, HbC, HbD, and HbE, which cause the sickling of red blood cells, red blood cell lifespan is reduced even further. In sickle cell disease (HbSS), for example, average RBC lifespan is just 10 to 20 days. “So what is the impact?” she asked. “Often it may just be a percent change to hemoglobin A1c.” That percent, however, has a considerable effect on average blood glucose values. A six percent HbA1c, for example, represents 126 mg/dL in average blood glucose. In contrast, an HbA1c of seven percent represents 154 mg/dL, and an eight percent HbA1c represents 183 mg/dL.
“So seemingly small differences could have significant implications for a diabetic patient,” she said.
The American Diabetes Association recommends that plasma blood glucose criteria, rather than HbA1c, be used to diagnose diabetes in patients with conditions associated with increased red blood cell turnover. And the National Institute of Diabetes and Digestive and Kidney Diseases says that physicians should not use the A1c test for patients with disease conditions such as HbSS, HbCC, or HbSC, she said, “noting that these values may not accurately represent the glycemic control of a patient.”
Dr. Rhea-McManus shared the case of a 36-year-old female of Sicilian descent with a history of obesity and hypertension. “She complained to her primary care physician that she was tired and often waking up at night to urinate.” At her appointment, her fasting glucose was 124 mg/dL—below the 126 mg/dL diagnostic threshold for diabetes. Her HbA1c, measured by immunoassay on two occasions, was 5.3 percent and 5.1 percent. Her physician assured her that she didn’t have diabetes and asked her to follow up in three months.
The patient then relocated to a different state and saw a new physician for an exam (four months after the prior appointment), during which her fasting glucose was 142 mg/dL. A subsequent two-hour oral glucose tolerance test was 220 mg/dL, “with 200 being the threshold for diagnosis with diabetes.” At this appointment, the patient’s HbA1c, performed by ion-exchange HPLC, was 16.7 percent. A note was appended to the lab report indicating that a presumptive hemoglobin variant was suspected.
Subsequent laboratory testing and investigation by the patient’s physician revealed the presence of HbS/beta thalassemia (Rhea JM, et al. Am J Clin Pathol. 2014;141[1]:5–16). “So using a method where you can’t identify or know that the patient has a hemoglobin variant could lead to delayed diabetes diagnosis, as in this case,” she said.
In another case, a 36-year-old Japanese male presented to the hospital with stroke. Though his fasting glucose was normal, at 120 mg/dL, his HbA1c was 10.1 percent, measured by immunoassay. “So he was started on medications to try to reduce that A1c value,” Dr. Rhea-McManus said. When he followed up with his physician, his fasting glucose was 73 mg/dL. “He had been tracking his home blood glucose levels and his logs showed that he was in good glycemic control, but his hemoglobin A1c was still 10 percent.” For the next several years the patient underwent adjustments to his medication, though he insisted his glucose logs were accurate and that he was compliant with his physician’s recommendations. After a couple of episodes of hypoglycemia, he was referred to a specialist. When his HbA1c was tested by HPLC it was 5.4 percent, and a note appended to the lab report indicated the presence of an abnormal peak. Subsequently he was found to have Hb Himeji, a rare and clinically silent Hb variant (Shimizu S, et al. J Jpn Diabetes Soc. 2015;58[2]:121–127).
“So in this case,” she said, “using a method in which there was no indication to the physician that there was a hemoglobin variant present led to the misdiagnosis of diabetes and two years of unnecessary treatment. After this finding he was removed from those medications and monitored, and his glucose stayed well within the normal ranges.”
In one study of 500 HbA1c samples submitted for testing in two hospitals, the authors determined the prevalence of HbSS (2.2 percent), HbSC (1.2 percent), and HbS/beta thalassemia (one percent) and estimated the number of samples for which a misleading HbA1c result would have been reported if assays unable to detect Hb variants were used. In 2011, for example, the total volume of HbA1c tested was 72,333 samples. Thus, an estimated 1,591 samples would contain HbSS, 868 would contain HbSC, and 723 would contain HbS/beta thalassemia (Rhea JM, et al. Arch Pathol Lab Med. 2013;137[12]:1788–1791). “These are just numbers that indicate the potential for reporting a clinically misleading hemoglobin A1c number,” Dr. Rhea-McManus said.
There needs to be good and open communication between the laboratory and the treating physicians, she said. “If the physician sees something that doesn’t make sense, they shouldn’t take the A1c value at face value—we should be able to have those conversations about what might be happening. And the lab can also help guide what the additional investigation should look like.”
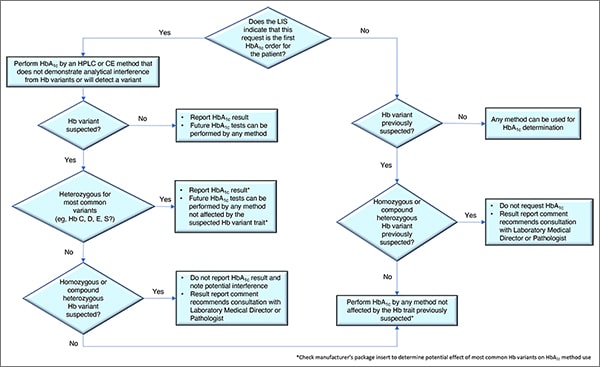
In Fig. 1 is a laboratory algorithm for HbA1c testing developed by Drs. Koch and Rhea-McManus and Lucia Berte. It begins with a question: Does the laboratory information system indicate this is the first HbA1c order for this patient? If yes, the test should be performed by an HPLC or capillary electrophoresis method that does not demonstrate analytical interference from the Hb variants or that can presumptively detect a Hb variant. If no presumptive Hb variant is suspected, the result can be reported and future tests can be performed by any method, Dr. Koch said. If a variant is suspected, and it’s identified as heterozygous for one of the most common variants, “we can report the hemoglobin A1c result,” but future tests should be performed using only methods not affected by the Hb variant trait. “Check the manufacturer’s package insert,” he said. “That will tell you whether or not they’ve identified these variants as being a factor to worry about.”
If no common variants are found, in some cases a homozygous or compound heterozygous Hb variant may be suspected. “If so, we should not report the hemoglobin A1c result,” he said, and a potential interference should be noted. Consultation by the provider with the laboratory medical director or a pathologist also is recommended.
If the request is not the patient’s first HbA1c order, “then we should know whether or not a hemoglobin variant has been previously suspected,” he said. If no variant has been suspected, any method can be used. If a variant was previously suspected and it’s thought to be heterozygous for one of the most common variants, any method not affected by the common heterozygous variants can be used. But if a variant was previously suspected, and it’s thought to be a homozygous or compound heterozygous variant, the requested result should not be reported and the report should recommend that a consultation take place with the laboratory medical director or pathologist.
If the LIS isn’t capable of identifying the patient’s past HbA1c result and whether a hemoglobin variant was present, it may be necessary to set up a prompt at the point of order to query the system for that information. This would involve coordinating with the IT department, “which is often challenging,” Dr. Koch said. “But it needs to be done.”
Charna Albert is CAP TODAY associate contributing editor.