Use of immunoglobulin gene clonality studies to facilitate diagnosis of unusual case
Thomas Shi, MD; Mohammad Alomari, MD; Ping Yang, PhD; Bekim Sadikovic, PhD; Nikhil Sangle, MD; Christopher J. Howlett, MD, PhD
 November 2017—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Western University and London Health Sciences Centre, London, Ontario, Canada. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
November 2017—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Western University and London Health Sciences Centre, London, Ontario, Canada. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Follicular lymphoma is an indolent, incurable, slow-progressing form of non-Hodgkin’s lymphoma. It is characterized by a proliferation of follicle center B-cells that usually express the t(14;18)(q32;q21) gene fusion, resulting in constitutive production of the BCL2 protein.1 Involvement of the gallbladder by follicular lymphoma is extremely rare. The first ever case reported in the English literature was in 2003,2 and since then there have been only sporadic reports. In a recent series of 14 cases of primary lymphomas of the gallbladder and extrahepatic bile duct, three of the 14 cases (21.4 percent) were follicular lymphoma.3
Here we report a case of follicular lymphoma of the gallbladder that required PCR testing using Biomed-2 primers for confirmation of diagnosis, as immunohistochemistry for BCL2 protein and FISH for IGH/BCL2 translocation were not conclusive. This case highlights the utility of PCR-based molecular studies when results from traditional diagnostic tests are inconclusive.
Case. A 55-year-old Caucasian female with unremarkable past medical history presented with symptoms of biliary colic and was found to have cholelithiasis. Laparoscopic cholecystectomy was performed without complication.
Grossly, the gallbladder wall was thickened (0.4–1.2 cm) with several scattered, raised areas on the gallbladder mucosa (0.3 cm in greatest dimension). Hematoxylin and eosin sections showed the gallbladder wall diffusely infiltrated by a lymphoid infiltrate (Fig. 1A). Several reactive-appearing secondary lymphoid follicles near the periphery were seen (Fig. 1A). However, the lymphoid follicles closer to the gallbladder mucosa appeared atypical, with non-polarized germinal centers and attenuated mantle zones and lacking tingible body macrophages (Fig. 1B). Immunohistochemical studies were performed and showed BCL6 positivity in the atypical follicles (Fig. 1F), with possible weak co-expression of BCL2 protein, but difficult to discern from intrafollicular small T-cells (Fig. 1H), while centrocytes in the reactive-appearing follicles were completely negative for BCL2 (Fig. 1G). CD20 staining nicely highlighted the polarized follicles and mantle zones (Fig. 1C), a feature not seen in the abnormal follicles (Fig. 1D). CD21 and CD23 showed slightly expanded follicular dendritic meshworks in atypical follicles. CD3 and CD5 stained the interfollicular T-lymphocytes. Ki67 showed a low proliferation index within the atypical follicles (Fig. 1J), as compared with the reactive follicles (Fig. 1I). Immunostains for kappa and lambda light chains were equivocal due to high background staining.
While the morphologic and immunohistochemical features described above were highly suspicious for partial involvement of the gallbladder by follicular lymphoma, we sought to clinch the diagnosis via cytogenetic or molecular studies or both. An interphase fluorescence in situ hybridization study failed to reveal evidence of IGH/BCL2 gene rearrangement; therefore, we proceeded to perform immunoglobulin heavy chain (IGH) and immunoglobulin kappa light chain (IGK) gene rearrangement studies using commercial Biomed-2 primers (Invivoscribe Technologies, San Diego). Briefly, genomic DNA was amplified by multiplex PCR containing fluorescently labeled primers that target the conserved framework (FR) and joining (J) regions of the immunoglobulin heavy chain IGH gene and the variable (V) and joining (J) and intragenic regions of the immunoglobulin kappa light chain IGK gene. Interestingly, the results from the IGH reactions revealed a polyclonal pattern; however, a definite monoclonal peak was observed in the IGK results (Fig. 1K), thus confirming the diagnosis of follicular lymphoma.
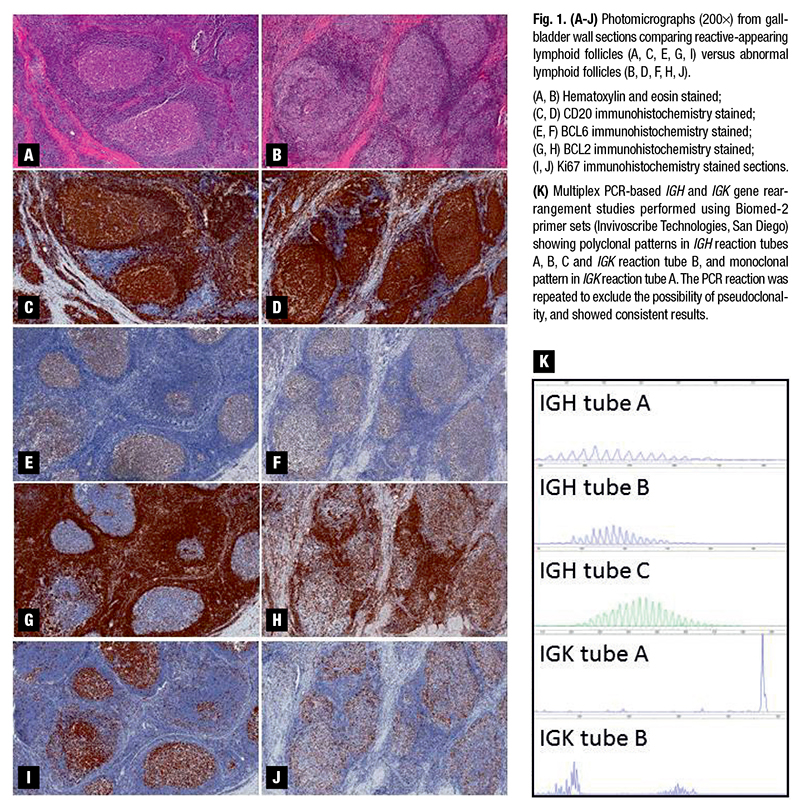 Discussion. This case report illustrates a stepwise approach in a rare case of gallbladder follicular lymphoma that uses molecular studies as a diagnostic aid. Despite the rare diagnosis, from a clinical standpoint the features of our case were in keeping with previously reported cases of gallbladder lymphoma,3 including female predilection, presenting symptoms of biliary colic with or without the presence of gallstone(s), and thickened gallbladder wall, though these are, of course, all nonspecific. From a histological standpoint there was clearly a population of abnormal follicles present. However, the lack of definitive BCL2 expression made it difficult to make a straightforward diagnosis of follicular lymphoma. Therefore, it was important to demonstrate cytogenetic or molecular evidence for confirmation.
Discussion. This case report illustrates a stepwise approach in a rare case of gallbladder follicular lymphoma that uses molecular studies as a diagnostic aid. Despite the rare diagnosis, from a clinical standpoint the features of our case were in keeping with previously reported cases of gallbladder lymphoma,3 including female predilection, presenting symptoms of biliary colic with or without the presence of gallstone(s), and thickened gallbladder wall, though these are, of course, all nonspecific. From a histological standpoint there was clearly a population of abnormal follicles present. However, the lack of definitive BCL2 expression made it difficult to make a straightforward diagnosis of follicular lymphoma. Therefore, it was important to demonstrate cytogenetic or molecular evidence for confirmation.
A FISH study did not reveal evidence of IGH/BCL2 translocation, and while this is unusual, it is known to occur in 12 percent to 15 percent of follicular lymphomas.1,4,5 While it is possible that this result was due to an alternative BCL2 translocation (IGH is the most common translocation partner, but IGK and IGL translocations with BCL2 also occur)5 or sampling error, it did correlate with the lack of significant BCL2 staining by immunohistochemistry, as follicular lymphomas with BCL2 translocation generally show strong BCL2 protein expression. Given our ongoing suspicion due to the abnormal morphologic features, we proceeded to use a molecular approach, looking for clonal IGH and/or IGK gene rearrangements. The results of this testing, which showed clonal rearrangements of IGK but not IGH genes, illustrate the added value of IGK studies over IGH studies alone, especially for germinal center and post-germinal center lymphomas, which may not reveal clonal IGH gene rearrangements. This is thought to be secondary to the effects of somatic hypermutation of IGH, which can result in nucleotide sequence changes that negatively affect primer annealing, thus giving false-negative results. In fact, studies have shown a sensitivity increase of up to 58 percent when clonality analysis of IGK is added over IGH alone in germinal center and post-germinal center lymphomas,6-9 due to a lesser frequency of somatic hypermutation in IGK versus IGH genes.9
Other molecular or cytogenetic testing strategies, which we did not pursue, may also be of use in difficult cases of follicular lymphoma. These include use of alternative FISH probes (e.g. break- apart probes for BCL2), PCR-based assay for IGH/BCL2 translocation, and/or FISH assay for BCL6 translocation, which has been observed in a subset of BCL2-negative cases of follicular lymphoma.10,11 False-negative immunohistochemistry for BCL2 secondary to mutation in the BCL2 gene has been reported in the literature.12 While it is possible that the lack of significant BCL2 staining in our case might be due to such a mechanism, it is more likely that ours is a bona fide BCL2-negative case of follicular lymphoma, given the negative FISH results.
In summary, we use a rare case of follicular lymphoma of the gallbladder to illustrate a rational stepwise approach to using morphology, immunohistochemistry, and molecular studies to confirm the diagnosis. While most cases of follicular lymphoma are straightforward and do not require molecular testing, there are occasional cases where it is helpful for confirmation. This case also illustrates potential pitfalls to molecular testing in follicular lymphoma, including the possibility of negative IGH/BCL2 FISH results and the potential for false-negative IGH gene rearrangement PCR studies.
- Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest. 2012;122(10):3424–3431.
- Ferluga D, Luzar B, Gadzijev EM. Follicular lymphoma of the gallbladder and extrahepatic bile ducts. Virchows Arch. 2003;442(2):136–140.
- Mani H, Climent F, Colomo L, Pittaluga S, Raffeld M, Jaffe ES. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. Am J Surg Pathol. 2010;34(9):1277–1286.
- Bernicot I, Douet-Guilbert N, Le Bris MJ, Herry A, Morel F, De Braekeleer M. Molecular cytogenetics of IGH rearrangements in non-Hodgkin B-cell lymphoma. Cytogenet Genome Res. 2007;118(2–4):345–352.
- Roullet M, Bagg A. The basis and rational use of molecular genetic testing in mature B-cell lymphomas. Adv Anat Pathol. 2010;17(5):333–358.
- Berget E, Helgeland L, Molven A, Vintermyr OK. Detection of clonality in follicular lymphoma using formalin-fixed, paraffin-embedded tissue samples and BIOMED-2 immunoglobulin primers. J Clin Pathol. 2011;64(1):37–41.
- Catherwood MA, Gonzalez D, Patton C, Dobbin E, Venkatraman L, Alexander HD. Improved clonality assessment in germinal centre/post-germinal centre non-Hodgkin’s lymphomas with high rates of somatic hypermutation. J Clin Pathol. 2007;60(5):524–528.
- Halldórsdóttir AM, Zehnbauer BA, Burack WR. Application of BIOMED-2 clonality assays to formalin-fixed paraffin embedded follicular lymphoma specimens: superior performance of the IGK assays compared to IGH for suboptimal specimens. Leuk Lymphoma. 2007;48(7):1338–1343.
- Payne K, Wright P, Grant JW, et al. BIOMED-2 PCR assays for IGK gene rearrangements are essential for B-cell clonality analysis in follicular lymphoma. Br J Haematol. 2011;155(1):84–92.
- Gu K, Fu K, Jain S, et al. t(14;18)-negative follicular lymphomas are associated with a high frequency of BCL6 rearrangement at the alternative breakpoint region. Mod Pathol. 2009;22(9):1251–1257.
- Guo Y, Karube K, Kawano R, et al. Bcl2-negative follicular lymphomas frequently have Bcl6 translocation and/or Bcl6 or p53 expression. Pathol Int. 2007;57(3):148–152.
- Schraders M, de Jong D, Kluin P, Groenen P, van Krieken H. Lack of Bcl-2 expression in follicular lymphoma may be caused by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol. 2005;205(3):329–335.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
