Swikrity U. Baskota, MD
January 2021—Rapid on-site evaluation (ROSE) for cytology specimens is performed at many institutions to improve the quality of health care by proper triage of obtained material to increase the diagnostic yield, or to direct appropriate investigation. It also helps to control health care costs by reducing the rate of nondiagnostic specimens, unnecessary passes, and repeat procedures. The number of procedures requiring ROSE is growing due to the increase in the number of platforms used to perform minimally invasive procedures. Since these procedures are often performed at locations distant from cytology laboratories, such as operating theaters and radiology, bronchoscopy, and endoscopy suites, cytology laboratory personnel spend a significant amount of time commuting. The ever-growing demands of these procedures seem to be outpacing the capacity of available cytology staff.
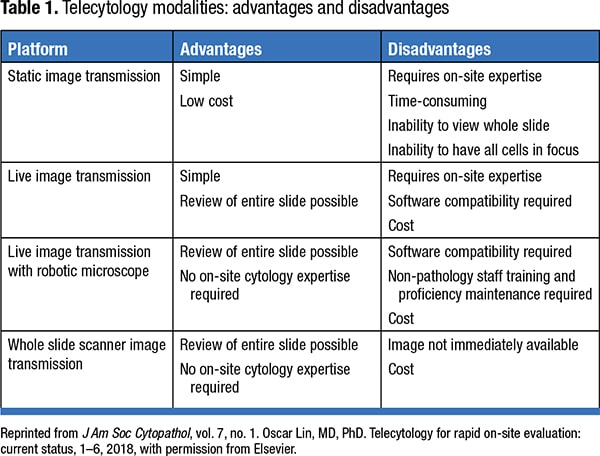 With COVID-19 social distancing guidelines implemented at the majority of the procedural suites, implementing telecytology might help to keep the number of people to a minimum at those sites. An article by Oscar Lin, MD, PhD, titled “Telecytology for rapid on-site evaluation: current status” (J Am Soc Cytopathol. 2018;7[1]:1–6), describes telecytology as a reasonable solution to increase the efficiency of the personnel who perform ROSE. In this article, he explains the need to include a pathologist in ROSE because of the complexity of cases and the billing requirements. The different types of image transmission that can be used for telecytology are static, live image streaming, and robotic live image streaming. Use of whole slide scanners is an option for the future. Their use is limited at this time due to technical challenges stemming from the uniqueness of cytologic preparations, such as the three-dimensional nature of cytology preparations requiring Z-stacking (multiple levels of scan) for proper visualization of cells, which in turn increases the turnaround time and cost.
With COVID-19 social distancing guidelines implemented at the majority of the procedural suites, implementing telecytology might help to keep the number of people to a minimum at those sites. An article by Oscar Lin, MD, PhD, titled “Telecytology for rapid on-site evaluation: current status” (J Am Soc Cytopathol. 2018;7[1]:1–6), describes telecytology as a reasonable solution to increase the efficiency of the personnel who perform ROSE. In this article, he explains the need to include a pathologist in ROSE because of the complexity of cases and the billing requirements. The different types of image transmission that can be used for telecytology are static, live image streaming, and robotic live image streaming. Use of whole slide scanners is an option for the future. Their use is limited at this time due to technical challenges stemming from the uniqueness of cytologic preparations, such as the three-dimensional nature of cytology preparations requiring Z-stacking (multiple levels of scan) for proper visualization of cells, which in turn increases the turnaround time and cost.
Table 1 highlights the telecytology platforms and their advantages and disadvantages. The selection of telecytology platform (hardware and software) should be based on an institution’s volume of cases, budget, provision of internet with appropriate bandwidth, and workflow.
Irrespective of the telecytology platform used, validating the clinical equipment to be used for clinical purposes in the U.S. is required. The validation should include the pathologist who is adequately trained to use the system and should follow the CAP guidelines for validating whole slide imaging for diagnostic purposes with a minimum set of 60 slides. The Centers for Medicare and Medicaid Services considers diagnostic examination of ROSE slides to be subject to compliance with CLIA regulations, and a CLIA number is required, except in states that are exempt from such regulations.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
