Amy Carpenter Aquino
July 2019—Qualitative HIV-2 RNA testing to resolve discordant HIV-2 results may be warranted but seldom results in confirmation of HIV-2 infection, Linda M. Styer, PhD, illustrated with data at the 2019 HIV Diagnostics Conference in March. She also presented an overview of eight years of HIV-2 viral load testing at New York State’s Wadsworth Center, where she is a research scientist in the Bloodborne Viruses Laboratory.
HIV-2 is rare and has its highest prevalence in West Africa. The most recent U.S. data (1987–2009) found that 66 percent of the 166 confirmed cases of HIV-2 in the United States were located in the Northeast, with 46 percent of those cases in New York City. “This is why in New York we are concerned about HIV-2,” said Dr. Styer, who is also an assistant clinical professor, Biomedical Sciences Department, School of Public Health, University at Albany, State University of New York.
Dr. Styer’s laboratory developed an HIV-2 real-time PCR assay to detect HIV-2 RNA and an internal control virus (mouse hepatitis virus) in serum or plasma. The assay starts with a lysis step and the addition of 0.1 to 0.9 mL of patient serum or plasma and 20 µL of the internal control virus. The total nucleic acid present in the samples is extracted using BioMérieux’s Nuclisens EasyMag extraction system, followed by a random hexamer reverse transcription reaction to generate cDNA, which is then amplified in a real-time PCR reaction.
The assay has two primer-TaqMan probe sets, Dr. Styer said. One detects the HIV-2 long terminal repeat region, which is present at both ends of the HIV-2 genome. The second set detects the internal control.
“We use a high-positive control, low-positive control, and negative control, and we also look at our internal control,” she said. The limit of detection for the highest volume of plasma used is seven international units (IU)/mL. A validation study was published in 2013 (Styer LM, et al. J Clin Virol. 2013;58[suppl 1]:e127–e133).
The HIV diagnostic testing algorithm recommended by the Centers for Disease Control and Prevention and the Association of Public Health Laboratories differentiates between HIV-1 and HIV-2 antibodies but indeterminate HIV-2 antibody results are possible. “This is where HIV-2 nucleic acid testing can be important,” Dr. Styer said.
One instance in which HIV-2 NAT can be useful is when there is unconfirmed reactivity to HIV-2 antibodies, which can occur when the Bio-Rad Geenius HIV 1/2 Supplemental assay provides a result of HIV indeterminate or HIV-2 indeterminate because of reactivity to only one of the two HIV-2 antigen bands (gp36 and gp140).
“It can also occur if you are running the Bio-Rad BioPlex 2200 HIV Ag-Ab assay where you have HIV-2 antibody reactivity but the Geenius HIV 1/2 Supplemental assay does not confirm that reactivity,” she said.
HIV-2 NAT is also useful in cases in which the result is HIV positive, untypable. “Generally, this result occurs because of cross-reactivity between HIV-1 and HIV-2 antibodies, which often resolves to reactivity to either HIV-1 or HIV-2 antibodies later on.”
It is also possible for a patient to be dually infected with HIV-1 and HIV-2, though this is extremely rare in the U.S.
Many public health laboratories lack access to HIV NAT because of the expense of maintaining a high-complexity test for the few specimens that require NAT, which can delay diagnosis and treatment (Wesolowski LG, et al. J Clin Virol. 2015;65:6–10).
The APHL/CDC HIV NAT Referral Project addressed this need by providing a mechanism for U.S. public health laboratories to send their HIV specimens that require NAT to one of two laboratories, the Bloodborne Viruses Laboratory at the Wadsworth Center or the Florida Bureau of Public Health Laboratories. In June 2016, the Wadsworth Center began offering HIV-2 RNA testing for all participating U.S. public health laboratories based on specific results: HIV antigen/antibody reactive followed by Geenius HIV-2 or HIV indeterminate, or a result on the BioPlex HIV Ag-Ab assay that was reactive for HIV-2 antibody but not confirmed by Geenius results (nonreactive or indeterminate).
The Wadsworth Center laboratory received 90 specimens for HIV-2 qualitative RNA testing between June 2016 and December 2018. Forty-eight specimens were submitted as part of the APHL/CDC Demonstration Project, and 42 specimens came from New York State clinicians, laboratories, and other contracted facilities.
Dr. Styer presented the test results in groups. Group one, the largest, contained 56 specimens with unconfirmed HIV-2 antibody reactivity. The results for 45 specimens in this group were HIV antigen/antibody reactive, Geenius HIV-2 indeterminate, and HIV-1 RNA not detected. “We did not detect HIV-2 RNA in any of those,” she said. “Unfortunately, we didn’t get the Geenius banding pattern on every one of those specimens so it’s hard to know what band was reactive in that HIV-2 indeterminate result.” For seven specimens they knew the banding pattern; all were gp140 (HIV-2 antigen) reactive.
Eight specimens were Geenius HIV indeterminate, and HIV-2 RNA was not detected in any of them. In two specimens the Geenius banding pattern was known: gp36 (HIV-2 antigen) and gp41 (HIV-1 antigen). Three specimens in group one had an undifferentiated result on the BioPlex HIV Ag-Ab assay and an HIV-2 antibody not confirmed result on the Geenius assay. No HIV-2 RNA was detected.
“There was one patient for whom we received five specimens that were all HIV-2 indeterminate on Geenius, and those specimens were collected over a 17-month period,” Dr. Styer said. “So there are some patients who have very long-term HIV-2 indeterminate results.”

Dr. Styer
Group two consisted of eight specimens on which HIV-2 nucleic acid testing was performed to rule out dual HIV-1, HIV-2 infections. All eight specimens were Geenius HIV-positive, untypable, and some had detectable HIV-1 RNA. “Once again we did not detect HIV-2 RNA in these specimens, suggesting that these are most likely cross-reactivity from people we are testing early in their infection process,” Dr. Styer said.
In group three, in which NAT was performed to “resolve HIV status,” there were 21 specimens in which the laboratory did not detect HIV-2 RNA. These specimens were submitted for HIV-2 RNA testing for multiple reasons: “Some were Geenius untypable in the initial test, but by the time we received the specimen for HIV-2 nucleic acid testing, it had resolved to HIV-1 positive,” Dr. Styer said.
One specimen was from a patient from Africa who was diagnosed as HIV-1 positive by Geenius but for whom no HIV-1 nucleic acid was detected in the HIV-1 viral load assay, so the aim was to rule out HIV-2. “We also had several specimens submitted from physicians who were confused about the Geenius result, which is a theme,” she said. “The individual analyte results were HIV-1 reactive, HIV-2 indeterminate, and they thought they needed to go on for HIV-2 nucleic acid testing when in fact they did not.” Specimens with these results have a final assay interpretation of HIV-1 positive on Geenius and should be referred to care for a HIV-1 infection, Dr. Styer tells CAP TODAY. According to the Geenius package insert, the HIV-2 indeterminate result in these specimens is “likely due to cross-reactivity of HIV-1 antibodies on HIV-2 antigens and confirmation of HIV-2 is not required.”
Other specimens were submitted to rule out HIV-2 infection in a pregnant patient from Africa and in a physician who exhibited symptoms similar to acute HIV infection after a needlestick injury received while treating a patient from Africa.
There’s another source of confusion with this category, Dr. Styer said. “Clinicians are confused about the detection ability of the HIV antigen/antibody test. They think it detects both HIV-1 and HIV-2 antigens. It only detects HIV-1 antigen, and therefore there is no way the initial HIV screening test can detect an acute HIV-2 infection.”
Group four consisted of five specimens submitted to confirm HIV-2 diagnosis. All were Geenius HIV-2 positive. “And aha! We did detect HIV-2 RNA, finally, in three out of the five specimens,” Dr. Styer said.
“We found that the HIV-2 RNA test helped exclude infection in specimens with unresolved HIV-2 antibody reactivity,” she said in summary. “The question is, are there too many unnecessary tests? Probably yes. Some could be cleared up if we got rid of the confusion about the detection ability of the antigen/antibody test.” Also leading to unnecessary tests are the misunderstanding around the Geenius results versus the interpretation, and false reactivity of the Geenius gp140 band.
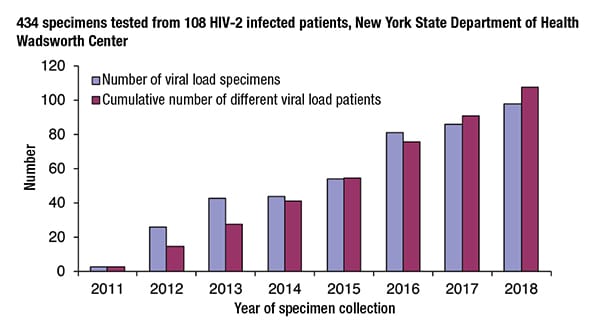
In her second session, Dr. Styer shared data to show that HIV-2 viral load testing is a valuable tool for monitoring treatment response and that while HIV-2 viral load values are generally thought to be low, values above 10,000 IU/mL are not uncommon.“Since 2011, we have tested 434 specimens from 108 HIV-2 infected patients,” Dr. Styer said. The Bloodborne Viruses Laboratory uses the same HIV-2 RT-PCR assay but with a few differences, such as testing only freshly collected plasma, using two new defined volumes of 0.2 and 0.9 mL, and incorporating a set of five standards with values set by the HIV-2 RNA international standard. “There are criteria for the linearity of that standard curve and also the predicted viral load values of the high and low positive controls.”
The assay also uses a no-reverse transcription control, which “allows us to detect HIV-2 DNA that may be contaminating our plasma samples,” Dr. Styer said. “Initially, we didn’t think we would continue using this control, but it has become useful.”
The HIV-2 viral load assay has the same limit of detection as the qualitative assay—7 IU/mL—but with a lower limit of quantification (41 IU/mL [0.9 mL], 185 IU/mL [0.2 mL]).
The number of viral load specimens the laboratory tests each year has increased consistently since 2011, with almost 100 specimens tested in 2018. And every year new viral load patients are added to the lab’s testing population. “It doesn’t look like we’re plateauing out yet. We anticipate that we will continue to add more patients as they are diagnosed,” Dr. Styer said.
The laboratory confirms each patient’s HIV-2 infection status with antibody testing, or with HIV-2 RNA testing for those for whom sufficient sample is not left over for antibody testing, which was the case for 23 of the 108 patients.
 Samples from an additional 47 patients were tested with Bio-Rad’s Multispot HIV-1/HIV-2 Rapid Test, which confirmed HIV-2 infection in 46 samples. “There was one HIV undifferentiated result, and it turned out to be a true dual infection,” Dr. Styer said. “We detected both HIV-1 and HIV-2 RNA.”
Samples from an additional 47 patients were tested with Bio-Rad’s Multispot HIV-1/HIV-2 Rapid Test, which confirmed HIV-2 infection in 46 samples. “There was one HIV undifferentiated result, and it turned out to be a true dual infection,” Dr. Styer said. “We detected both HIV-1 and HIV-2 RNA.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
