December 2019—At Mayo Clinic, the latest generation of cardiac troponin assay was an overnight success. Literally. For months, the institution had been preparing to switch to Roche Diagnostics’ Elecsys Troponin T Gen 5 Stat assay, says Bradley S. Karon, MD, PhD, chair of the Division of Clinical Core Laboratory Services, Department of Laboratory Medicine and Pathology. As the time of the rollout neared, an internal medicine colleague who was involved in overseeing the transition asked, “‘What will the burn-in period be?’” recalls Dr. Karon, who is also co-director of Mayo’s stat labs and point-of-care testing programs. Surely clinicians would be able to continue ordering the Gen 4 assay for a time, right? So how long would that last? Dr. Karon’s dramatic-sounding answer? “Zero hours.”
Read More »2019 Issues
For gestational diabetes, one step or two?
December 2019—The controversy surrounding the approved methods for screening gestational diabetes mellitus took the form of a debate at this year’s AACC annual meeting, with two speakers defending the one-step or two-step method.
Read More »Labs size up options for unpredictable flu
December 2019—There aren’t good flu seasons; there are just varying degrees of how bad they are. That’s the message the CDC wants people to hear, says Lynnette Brammer, MPH, lead for the CDC’s domestic influenza surveillance team. It’s what laboratories know well and why platforms, panels, and prescribing patterns are top of mind.
Read More »Dodging point-of-care testing potholes in PT, IQCP
December 2019—For point-of-care testing, perform proficiency testing on only one method or instrument unless your testing procedure says all patient samples must be tested on multiple instruments. And if a single IQCP is written for more than one POC testing location, account for all variations.
Read More »Millions at stake in ’21; CAP fights Medicare cuts
December 2019—Medicare reimbursement to pathologists in 2020 is estimated to remain steady, but significant cuts in payments to pathologists and other specialists are expected in 2021 owing to a dramatic shift in how primary care physicians will be paid. The CAP is already fighting the scheduled cuts to pathologists in 2021 as a result of a new plan that reimburses evaluation and management office visit services at a higher rate and lowers payments for non-E/M services billed by specialists.
Read More »LIS roundtable: The conversation continues—consolidation, IT labor force
December 2019—IT as it relates to laboratory consolidation and the labor supply for lab IT were some of what came up when CAP TODAY publisher Bob McGonnagle convened a panel in September to talk about laboratory information systems. Part one of the discussion is in the November issue (with the LIS product guide); part two begins here. On the panel were J. Mark Tuthill, MD, of Henry Ford Health System, Curt Johnson of Orchard Software, Wally Soufi of NovoPath, Michelle Del Guercio of Sunquest Information Systems, Nick Trentadue of Epic, Sepehr Seyedzadeh of Siemens Healthineers, and Tony Barresi of Beckman Coulter.
Read More »Diagnosing GDM in the first trimester
December 2019—“If you thought that diagnosing gestational diabetes at 24 to 28 weeks was unsettled, you haven’t seen anything yet.” That was David B. Sacks, MB, ChB, of the National Institutes of Health, speaking this year in the AACC session on gestational diabetes mellitus (GDM) with his co-presenters who debated the use of the one-step and two-step methods for diagnosing GDM in the second and third trimesters (see story, page 1). His talk: “Let’s Not Wait: Diagnosing GDM in the First Trimester.”
Read More »Panel explores urinalysis solutions, rules, POC testing
December 2019—What do users of urinalysis systems want? According to those in the know, the answer is instruments that are scalable and modular, maximize automation, reduce hands-on time, improve workflow, and more. CAP TODAY publisher Bob McGonnagle convened a panel in October to discuss these topics and other aspects of urinalysis testing. On the panel were Megan Nakashima, MD, of Cleveland Clinic; Michelle Dumonceaux, of Beckman Coulter; Maya Daaboul, of Siemens Healthineers; and Jason Anderson, MPH, MT(ASCP), of Sysmex. What they said follows.
Read More »AMP case report: Identification of a single exon deletion using NGS in a patient with Perlman syndrome
December 2019—We report a case of a 34-week-gestation male infant with prenatal overgrowth born to a 19-year-old G1P0 mother. No significant family history or consanguinity were reported. The infant was delivered emergently by cesarean section due to pregnancy-induced hypertension and polyhydramnios.
Read More »Reflections on biomarkers: so far along, so far to go
December 2019—Presentations at the recent Cancer Biomarkers Conference IV, sponsored by the University of Mississippi Medical Center, prompt reflection on how far we have come since the introduction of Rituxan, the first monoclonal antibody to be approved by the Food and Drug Administration for the treatment of cancer in 1996. Before the launch of Rituxan, physicians were skeptical about the efficacy and safety of monoclonal antibody therapy because of the history of failures.
Read More »Three inside one: biobank, CRO, reference lab
December 2019—Don’t even try to put Boca Biolistics into a box. The Pompano Beach, Fla., company is the rare outside-the-box model of three levels of service under one roof: an expansive biorepository, a contract research organization, and a reference laboratory that earned CAP accreditation in March. Joseph Mauro, president and CEO of Boca Biolistics, has been developing the “new” model for two decades. “The thing that makes us different is that we’re a vertically integrated company encompassing a biobank, CRO, and reference lab. There aren’t many players in this space. LabCorp and Quest have been buying CRO companies, so they are moving into that area, but they are not biobanks. They do not have all of the platforms we have.”
Read More »Put It on the Board
FDA grants de novo designation to NGS HIV-1 genotyping assay
December 2019—Vela Diagnostics received FDA authorization to market its in vitro diagnostic test for the detection of HIV-1 genomic drug resistance mutations. The Sentosa SQ HIV-1 Genotyping Assay uses the plasma of patients infected with HIV-1 to detect HIV-1 Group M drug resistance mutations in the protease, reverse transcriptase, and integrase regions of the pol gene, in a single test. It is the first HIV-1 genotyping NGS assay to receive marketing authorization from the FDA. In a Nov. 5 statement, Vela acting CEO and chairman of the board Sam Dajani called the FDA’s granting of the de novo designation to the NGS assay “a major milestone in HIV diagnostics.” “With the Sentosa SQ HIV-1 Genotyping Assay, laboratories will now have a sample-to-report solution to aid in monitoring and treating HIV-1 infection,” he said. The assay is validated on the Sentosa NGS workflow that enables automated RNA extraction, PCR setup, library construction, template preparation, sequencing, data analysis, and automated reporting.
Q&A column
Q. How do you report the presence of immature granulocytes in a 100-cell differential? Read answer. Q. Should a patient with a hematocrit greater than 55 percent be redrawn for correction always or only when prothrombin time and partial prothrombin time are elevated? Read answer.
Read More »From the President’s Desk: Looking ahead to 2020
December 2019—This time of year, it’s easy to find ourselves caught up in holiday planning and the challenges of managing a busy team’s hectic vacation schedule. But it is also the appropriate time to look ahead to the coming year and think about the opportunities as well as the challenges we should expect. Here’s what I can say for sure about next year and the years after that: The importance of pathologists and the laboratories that we direct will increase. Already, the majority of diagnoses and therapies are greatly influenced, if not outright dictated, by pathologists and our laboratories.
Read More »Newsbytes
How a local startup solved a lab’s slide-management issues December 2019—When Alex Bushell, the young CEO of a Canadian startup, approached Bernard Schaan, the now-retired laboratory manager at Peterborough Regional Health Centre, in 2017, to ask what problem they might tackle together, Schaan mentioned an issue that had nagged him for years: histology slide filing and retrieval. Tasked with filing between 125,000 and 135,000 slides per year, “I thought the process could be automated and had been asking various sales reps if there’s anything out there—and they said no, there wasn’t,” Schaan says.
Read More »Clinical pathology selected abstracts
Intraoperative red blood cell transfusion and mortality after cardiac surgery
December 2019—Patients with underlying cardiac disease are at risk for myocardial ischemia if they have untreated anemia at the time of cardiac surgery. During surgery, ongoing blood loss and hemodilution as a result of cardiopulmonary bypass (CPB) cause low hemoglobin levels. The optimal transfusion trigger for cardiac surgery patients continues to be debated. A more restrictive RBC transfusion strategy is used in patients with stable cardiovascular disease and is considered safe.
Anatomic pathology selected abstracts
Evaluation of tumor quantitation as an aid in predicting biochemical recurrence in organ-confined prostate cancer
December 2019—In the eighth edition of the AJCC Cancer Staging Manual, all organ-confined disease is assigned pathologic stage T2, without subclassification. The authors investigated whether total tumor volume (TTV) or maximum tumor diameter (MTD) of the index lesion, or both, enhance the ability to predict biochemical recurrence in pT2 patients. They identified 1,657 patients using digital tumor maps and quantification of TTV/MTD who had pT2 disease on radical prostatectomy.
Molecular pathology selected abstracts
Personalized oligonucleotide therapy for treatment of a rare genetic disease December 2019—A variety of molecular diagnostic laboratory tools are available to diagnose diseases caused by mutations in the human genome. However, few treatments are available to correct the underlying pathophysiology driven by these mutations. This is due, in part, to pharmaceutical companies’ inability to justify, from a business perspective, the expense and time necessary to develop and obtain FDA approval for novel therapies that benefit only a small number of patients.
Read More »Mate pair sequencing yields rich new data
November 2019—The LUVOIR telescope proposed this year by NASA, when it is launched into orbit, will outperform the Hubble Telescope 40-fold in ability to detect and visualize deep space objects in detail. But while dazzling in concept, the LUVOIR is still in development. Interestingly, at the genomic level, a similarly impressive advance in detection called mate pair sequencing has already progressed from research to clinical use in diagnosing cancer. With mate pair sequencing, a novel next-generation sequencing technique, Mayo Clinic is advancing the laboratory’s current capabilities for visualizing genetic rearrangements, thus increasing the diagnostic yield of testing for a variety of neoplasms.
Read More »Transgender adult reference intervals taking shape
November 2019—Current sex-specific intervals can be used to interpret hematology results for transgender people using their affirmed gender, say authors of a study published earlier this year.
Read More »Personnel paradox and more: POC pitfalls
November 2019—Point-of-care testing makes up only about 10 percent of all laboratory testing but the aggravation factor and number of people involved far exceeds that, said Deborah A. Perry, MD, medical director of pathology at Methodist Hospital in Omaha, Neb., speaking at CAP19 and calling POC testing “a whole different world.”
Read More »NTRK fusion testing: ups, downs of four methods
November 2019—With two inhibitors approved by the FDA for the treatment of NTRK-fusion-positive solid tumors, the next step is to determine whom to test and how. If the efficacy of the compounds—larotrectinib and entrectinib—were the only thing to consider in implementing a testing algorithm, knowing whom to test would be easy.
Read More »In checklists, new approach for predictive markers
November 2019—In the 2019 edition of the CAP accreditation program checklists, released in September, requirements for predictive marker testing were revised to make them general so they apply to all types of such testing performed by immunohistochemistry and in situ hybridization, wherever possible.
Read More »HemoCell workcell approach brings efficiencies to coag
November 2019—Total laboratory automation solutions, with their integrated, comprehensive approach, have meshed well with the goals of many central labs. But with HemoCell, the first lab automation solution designed for hemostasis testing, Instrumentation Laboratory has shifted gears toward a more specialized solution: a workcell to improve quality and efficiency through process standardization. IL’s HemoCell integrates the company’s ACL Top 750 LAS testing systems, HemoHub Intelligent Data Manager, and HemosIL reagents with Thermo Fisher Scientific’s TCAutomation track. The company’s initial customer base for HemoCell has been in Europe, Asia (particularly China), and South America. Now IL hopes to bring the benefits of HemoCell to more U.S. labs.
Read More »LIS panel talks middleware, wish lists, workflow
November 2019—Middleware, result reporting workflow, consolidation of labs, and IT and laboratory labor were the center of discussion when CAP TODAY publisher Bob McGonnagle convened a panel in September to talk about lab information systems. Part one of the roundtable begins here; part two, on consolidation and labor, will be published in the December issue. On the panel were J. Mark Tuthill, MD, of Henry Ford Health System, Curt Johnson of Orchard Software, Wally Soufi of NovoPath, Michelle Del Guercio of Sunquest Information Systems, Nick Trentadue of Epic, Sepehr Seyedzadeh of Siemens Healthineers, and Tony Barresi of Beckman Coulter.
Read More »Put It on the Board
Illumina and Qiagen partner to deliver sequencing-based IVD tests
November 2019—Illumina announced on Oct. 7 a 15-year partnership intended to broaden the availability and use of NGS-based in vitro diagnostic kits, including companion diagnostics. The agreement grants Qiagen nonexclusive rights to develop and globally commercialize IVD kits to be used with Illumina’s MiSeq Dx and NextSeq 550Dx systems. The agreement also includes rights for expansion of the partnership on future Illumina diagnostic systems. Both partners are also exploring opportunities for Qiagen to develop and market companion diagnostics based on Illumina’s TruSight Oncology assays. Illumina and Qiagen will cooperate to commercialize a menu of clinically validated workflows that combine Qiagen’s proprietary content and bioinformatics solutions. The partnership will focus initially on commercializing oncology IVD kits and may expand in the future to include additional clinical diagnostic fields, such as cardiology, hereditary diseases, infectious diseases, and inflammatory and autoimmune diseases.
Q&A column
Q. Does a histotechnologist need a bachelor’s degree to run in situ hybridization? Read answer. Q. Does using an alcohol swab affect the results of an ethanol blood test? Read answer. Q. Is there a recommended procedure for or reference article about checking APTT reagent sensitivities (for the identification of factors VIII and IX) when changing lot numbers and reference range? Read answer.
Read More »From the President’s Desk: Small steps, big impact
November 2019—There is a saying about Washington that applies to our state capitals as well: If you are not at the table, you are on the table. I want to spend this column considering some of the ways in which the CAP helps its members get off the plate and pick up a fork. In other words, to become engaged politically.
Read More »Newsbytes
Francisco Partners to acquire Orchard Software
November 2019—Francisco Partners, a technology-focused private equity firm, announced Sept. 30 its intent to acquire Orchard Software. CAP TODAY publisher Bob McGonnagle, on Oct. 2, spoke with Orchard founder and CEO Rob Bush and with Billie Whitehurst, who will succeed Bush as CEO. Whitehurst most recently was senior vice president at Netsmart. Here is what they, and Curt Johnson and Kerry Foster, of Orchard, had to say about the acquisition.
Letters
November 2019—We read with interest the case described by Vormittag-Nocito, et al., of metastatic bladder adenocarcinoma with associated molecular pathology findings (September 2019). However, we would like to point out what we believe to be an important update related to the significance of FGFR3 alterations in urothelial carcinoma. When referring to TP53 and FGFR3 alterations in urothelial carcinoma, the authors write that “no targeted therapy or prognostic implications can be made about these mutations at this time.”
Read More »Clinical pathology selected abstracts
Intensive versus standard blood pressure control with cerebral white matter lesions
November 2019—The effect of intensive systolic blood pressure control on brain health is uncertain, despite its efficacy for reducing cardiovascular morbidity and mortality. However, hypertension is a primary risk factor for cerebral small vessel ischemic disease (SVID), especially in developing white matter lesions (WML). The pathogenesis of Alzheimer disease and related dementia is known to be associated with SVID and cognitive decline.
Anatomic pathology selected abstracts
Angiosarcoma of the liver: clinicopathologic features and morphologic patterns
November 2019—Angiosarcoma is a rare malignant neoplasm of the liver for which morphologic patterns have not been systematically studied. To provide more comprehensive data on morphologic patterns, the authors reviewed angiosarcomas that had been diagnosed between 1996 and 2016 at a large medical referral center. The major growth patterns were classified as sinusoidal (nonmass forming) or mass forming.
Molecular pathology selected abstracts
Tumor microbiome diversity and composition: influence on pancreatic cancer outcomes November 2019—Pancreatic adenocarcinoma has a dismal prognosis, with a high incidence of relapse and a median overall survival of 24 to 30 months. Only nine percent of patients are alive five years after surgery. Genome-wide mutational landscape studies to decipher the factors that contribute to long-term survival have been futile. Recent studies in patients with melanoma and lung cancer have shown that the gut microbiota can mediate tumor responses to chemotherapy and immunotherapy, influencing overall outcome.
Read More »Catching CKD sooner with kidney profile
October 2019—Rarely (as waggish folks like to remind us) is it necessary to reinvent the wheel. Many times it’s better to take existing wheels and stick them on, say, a suitcase. Suddenly, maneuvering through airports becomes 1,000 times easier. Improvement can be that simple, so obvious in retrospect. At least that’s what kidney experts, both inside and outside the laboratory, are hoping as they promote use of the kidney profile lab order to diagnose and monitor chronic kidney disease. Transformation doesn’t always require the thrill of the new. While new markers are always welcome, two stalwart tests—estimated glomerular filtration rate and urine albumin-creatinine ratio—can do plenty.
Read More »For GI cancer, a digital and molecular reset
October 2019—You may not be curling up next to the fire with a cup of hot chocolate to read your copy of the new Digestive System Tumours, part of the World Health Organization Classification of Tumours Series.
Read More »Reporting, other changes in requirements for histocompatibility labs
October 2019—Histocompatibility laboratories that provide services for cellular therapy transplant patients have one more inspection option to consider, thanks to a new edition of the CAP’s histocompatibility checklist released in September.
Read More »For accredited biobanks, a path to CLIA equivalence
October 2019—The requirement revisions in the new edition of the Biorepository Accreditation Program checklist, published last month, are aimed at accommodating a growing overlap between clinical diagnostic activity and biomedical research.
Read More »Faster diagnosis? Chlorinated lipids in sepsis
October 2019—Chlorinated lipids have been shown to be new potential biomarkers for sepsis, and continuing research into their role could lead to faster diagnosis, said David A. Ford, PhD, of Saint Louis University School of Medicine, at this year’s AACC annual meeting. Dr. Ford, a professor of biochemistry and molecular biology, discovered chlorinated lipids in 2002, and at the AACC meeting he shared recent research on the association between chlorinated lipids and lung injury and death in sepsis patients.
Read More »Using predictive analytics to gauge sepsis risk
October 2019—How well can analytics predict the risk for sepsis? T. Scott Isbell, PhD, DABCC, director of clinical chemistry and point-of-care testing, SSM Health, Saint Louis University Hospital, at this year’s AACC annual meeting shared his hospital’s experi-ence with Epic’s sepsis predictive tool. Launched in 2017, the tool uses predictive analytics to produce a sepsis score for ad-mitted patients based on regular scans of key data elements in the electronic health record.
Read More »AMP case report: Use of MYD88 sequencing to confirm diagnosis of PIOL in a case with limited sample availability
October 2019—Primary intraocular lymphoma (PIOL) is a rare but aggressive B-cell malignancy usually considered as a subtype of primary central nervous system lymphoma. The most common form of PIOL is primary vitreoretinal lymphoma. PIOL is also known as the masquerade syndrome because it frequently mimics other ocular conditions such as chronic uveitis, which may be steroid-resistant. Its diagnosis is challenging and requires a high degree of suspicion. Here, we present a case of PIOL, the diagnosis of which was clinched based on the identification of a mutation in the myeloid differentiation factor 88 (MYD88) gene.
Read More »Hematology panel: bridging gaps, staffing, Lab 2.0
October 2019—Automation, the workforce shortage, manual review rates, and Laboratory 2.0 were some of what came up in CAP TODAY’s latest gathering of hematology experts for a roundtable on what’s new, pressing, and in play. CAP TODAY publisher Bob McGonnagle convened a panel in August consisting of Cordelia Sever, MD, of TriCore Reference Laboratories; Olga Pozdnyakova, MD, PhD, of Brigham and Women’s Hospital; Danette Godfrey and Simon Shorter of Sysmex; and Matt Rhyner, PhD, MBA, and Rachel Burnside, PhD, MBA, of Beckman Coulter. What they said follows.
Read More »With real-time data analytics, lab drills down to step it up
October 2019—As payments to laboratories decline and labs look for costs to cut, drawing on Lean and CAP 15189 know-how is the path to stronger productivity, workflow, and quality, “and all of that is eventually going to help,” says Mike Black, MBA, MT(ASCP), DLM, laboratory assistant VP of Avera McKennan Hospital and University Health Center, Sioux Falls, SD, and Avera laboratory service line administrator.
Read More »Put It on the Board
Sysmex joins Lab 2.0
October 2019—Sysmex America has joined the Clinical Lab 2.0 movement to support collaboration around value-based health care. The company said it is providing a grant focused on a multi-institutional demonstration project to develop actionable clinical strategies for anemia early detection, intervention, and prevention. Clinical Lab 2.0 is a Project Santa Fe Foundation initiative established to help develop the evidence base for the valuation of clinical laboratory services. It is a call for laboratory leadership in managing population health and enabling value-based care to evolve. Sysmex is the first corporate sponsor. “The Clinical Lab 2.0 movement, with its critical measurable and actionable attributes, promotes the clinical and business model of the future for clinical laboratories,” Khosrow Shotorbani, president and executive director of Project Santa Fe Foundation, said in a statement. The third annual Clinical Lab 2.0 Workshop will take place Nov. 3–5 in Chicago (www.cl2lab.org/clinical-lab-2-0-3rd-annual-workshop-registration-2-2/).
Q&A column
Q. What are your recommendations for using viscoelastic assays to perform platelet mapping studies? What is the clinical value of obtaining these test results? Read answer. Q. Would the results of tests for routine and special coagulation studies be affected if I thawed frozen plasma samples using a dry heating block? Are we allowed to use dry heating blocks? Read answer.
Read More »From the President’s Desk: Why you need to be active in the CAP
October 2019—I have two goals as a pathologist, and I suspect that my colleagues share them. First, I want to be the best physician—the best pathologist—so I can give the best possible care to my patients. Second, I want to get paid fairly for the service I provide. Being the best pathologist comes with a number of requirements: staying up to date on the latest information and protocols, practicing in a quality lab, and working with great pathologists.
Read More »Newsbytes
Digital health education: imperfect to imperative: October 2019—Arlen Meyers, MD, MBA, is a passionate advocate for educating medical students and practicing physicians about digital health technologies and their role in patient care. Without increased emphasis on organized digital health education, the medical field cannot fully embrace such technologies, says Dr. Meyers, president and CEO of the Society of Physician Entrepreneurs and co-editor of Digital Health Entrepreneurship, released this year by Springer Books.
Read More »Clinical pathology selected abstracts
Cardiovascular events and mortality in white coat hypertension
October 2019—Hypertension is the most common preventable cause of disability and premature mortality worldwide. It is often diagnosed using in-office blood pressure measurements. More recent guidelines encourage out-of-office blood pressure monitoring, such as at-home self-monitoring, for diagnosing and managing hypertension.
Anatomic pathology selected abstracts
Keratin 17: a sensitive and specific biomarker of urothelial neoplasia
October 2019—There is a clinical need to identify novel biomarkers to improve diagnostic accuracy for detecting urothelial tumors. The authors conducted a study to evaluate keratin 17 (K17), an oncoprotein that drives cell cycle progression in cancers of multiple anatomic sites, as a diagnostic biomarker of urothelial neoplasia in bladder biopsies and urine cytology specimens. The authors evaluated K17 expression using IHC in formalin-fixed, paraffin-embedded tissue specimens of nonpapillary invasive urothelial carcinoma (UC; classical histological cases), high-grade papillary UC (PUC-HG), low-grade papillary UC (PUC-LG), papillary urothelial neoplasia of low malignant potential (PUNLMP), and normal bladder mucosa.
Molecular pathology selected abstracts
Circulating tumor DNA as a clinical test in resected pancreatic cancer
October 2019—Pancreatic ductal adenocarcinomas are associated with high rates of mortality due, in part, to a lack of effective screening strategies and advanced disease at diagnosis. Residual occult disease is thought to contribute to disease recurrence in up to 80 percent of patients treated surgically for localized disease. These findings highlight the critical need for biomarkers for detecting disease early and monitoring tumor dynamics. Current strategies involve a combination of serum markers (carbohydrate antigen [CA] 19–9) and imaging modalities, both of which have limitations, particularly for detecting early disease recurrence postoperatively.
AP labs diving deeper into automation
September 2019—Talk to a few anatomic pathology laboratory directors about automation, and you may hear references to early network television, when automation’s downsides were mined for comedy. In one interview with CAP TODAY, a lab director drew parallels between potential backups in the automated AP lab and Lucy and Ethel’s travails keeping pace with a chocolate factory conveyor belt. There is strong agreement, to state it in 1960s TV terms, that even for core or centralized AP labs with the necessary volume, the traditional automation options have progressed well beyond the modern Stone Age, but reaching Tomorrowland may require a shift in thinking.
Read More »Shifting trends test labs’ financial mettle
September 2019—There’s nothing new about lab and pathology reimbursement cuts that threaten bottom lines. Over the past three or four decades they’ve become essentially part of the wallpaper in health care.
Read More »New requirements for molecular micro waived testing
September 2019—Four new checklist requirements for waived molecular-based microbiology tests have been added to the CAP point-of-care testing, limited service laboratory, and immunology accreditation program checklists, as part of the 2019 checklist edition released this month.
Read More »Laboratory 2.0: teaming up against opioid use disorder
September 2019—Laboratory pharmacists are a rare breed. When Monique Dodd, PharmD, PhC, MLS(ASCP), spoke at the Executive War College this year, she joked that a third of the laboratory pharmacists in the country might be in the audience.
Read More »Better than glass slides? Digital pathology’s challenge
September 2019—The FDA’s clearance this year of a second digital pathology system for primary diagnosis is likely to lead to lower costs and greater innovation and interoperability, say digital pathology advocates. A multicenter study led the FDA in May to clear Leica Biosystems’ Aperio AT2 DX System for clinical diagnosis in the United States.
Read More »Up-front on PAMA impact, private payer pricing
September 2019—I’m going to talk about our experience over the past couple of years with PAMA, which seeks to produce market pricing, and some of the lessons we’ve learned. We all know how we got here and one of the questions has always been: Did we ever have market pricing? The answer is probably not. When you’re dependent on a 30-plus-year-old fee schedule that never was revised for technology revisions, there wasn’t market pricing to begin with. And at the end of all of what we’re experiencing, we still don’t quite have it.
Read More »Rapid assay detects HIV, syphilis simultaneously
September 2019—The results of a clinical trial of a rapid assay for dual detection of HIV and syphilis were reported at the 2019 HIV Diagnostics Conference in March. If approved by the FDA, where it has been under consideration since 2018, the Chembio Diagnostic Systems assay would be the first point-of-care assay of its kind on the U.S. market.
Read More »AMP case report: NGS of a rare metastatic bladder adenocarcinoma
September 2019—Primary bladder adenocarcinoma is a rare vesicle malignancy accounting for up to two percent of malignant neoplasms of the bladder.1 They occur in males more than females and are classically seen in the fifth or sixth decade of life.2 Histologically they are of enteric, mucinous, or mixed types. Morphologically, the enteric type appears identical to a colonic adenocarcinoma and the mucinous type appears as neoplastic cells floating in pools of extravasated mucin. The mixed type is a mixture of the morphologies of the enteric and mucinous types. Immunohistochemically, adenocarcinomas of the urinary bladder classically express CK20 and CDX2.
Read More »Put It on the Board
AACC panel votes Inflammatix technology most disruptive
September 2019—Inflammatix, a molecular diagnostics company, received the American Association for Clinical Chemistry’s Disruptive Technology Award at the AACC annual meeting in August. The company, in Burlingame, Calif., won for its rapid HostDx tests. The award was based on Inflammatix’s presentation during the meeting. It was one of three finalists selected to present to a panel of expert judges during a special session. “We’re developing rapid tests that read the immune system to improve disease diagnosis and are initially focused on developing tests for acute infections and sepsis, which are two of the biggest public health challenges of our time,” Tim Sweeney, MD, PhD, Inflammatix cofounder and chief executive officer, told CAP TODAY. The company’s first test, HostDx Sepsis, measures the expression of multiple immune genes to determine rapidly if a patient has a bacterial or viral infection and whether the patient has or is likely to develop sepsis, Dr. Sweeney said.
Q&A column
Q. Is there clinical value in doing routine manual band counts to detect infection in newborns, especially since procalcitonin and immature neutrophil counts are available? Read answer. Q. Can CoQ10 supplements affect the level of Coumadin (that is, the INR)? Read answer.
Read More »From the President’s Desk: A summit, a park, and parting words
September 2019—The CAP will sponsor next May the Pathologists Leadership Summit in Washington, DC. This is not just another meeting where you’ll learn to deal with changes happening around you that feel as if they are out of your control. No. This is a radical reinvention of a meeting where members will come together to achieve something specific—and move pathology forward.
Read More »Newsbytes
September 2019—It’s a simple and nearly airtight communication strategy: Tell someone something verbally and then share the same message with them in writing to make sure they understood you. Following this logic, a group of surgical pathologists at the University of Minnesota Medical Center made an assumption that if their intraoperative consultation results were made available to surgeons in written form during surgery as documentation of verbal communication—either in person or via telephone—the frequency of communication errors would be reduced.
Read More »Clinical pathology selected abstracts
September 2019—Pathologists play a critical role in patient diagnosis, and a shortage of pathologists may result in overwork, diminished quality of work, diagnostic errors, and delay in diagnosis. The authors of this study examined trends in the total U.S. pathologist workforce, using the Canadian pathologist workforce as a reference.
Read More »Anatomic pathology selected abstracts
Validation of 2016 ITBCC recommendations for tumor budding in stages I–IV colorectal cancer
September 2019—Tumor budding is a robust prognostic parameter in colorectal cancer and can be used as an additional factor to guide patient management. Although backed by large bodies of data, a standardized scoring method is essential for integrating tumor budding into reporting protocols.
Molecular pathology selected abstracts
September 2019—The pathologist’s ability to interpret the complex spatial organization within and between cells and intercellular matrices is the basic underlying principle of morphologic pathology. Even in the genomic era, molecular genetic information is not clinically useful without tissue context. Modern spatial capturing methods, either by low-fidelity light microscopy or high-fidelity electron microscopy, cannot concomitantly interrogate a nucleic acid sequence.
Read More »Microbiome swims into our ken
August 2019—Even though he practices in Houston, James Versalovic, MD, PhD, swears he can see the coastline. As he and colleagues at Texas Children’s Hospital delve into research related to the human microbiome, several diagnostic and treatment possibilities are starting to appear tantalizingly close. Comparing their endeavors to ocean explorers of yore, he says, “We’re not quite sure exactly what is in front of us. We can see land—but we’re not quite there.” But unlike Captain Cook and company, Dr. Versalovic and others in the field have access to next-generation sequencing (though Dr. Versalovic jokes, “It’s been around long enough to be called this-generation sequencing”).
Read More »PCT leads the way in antimicrobial stewardship
August 2019—Antibiotic treatment of sepsis patients often has to rely on clinical observation and educated guesswork as clinicians wait for a culture to determine whether the infection is bacterial, viral, or possibly fungal.
Read More »CDC reports on two alternative HIV testing algorithms
August 2019—For HIV testing, a three-step algorithm that differs from the one recommended since 2014 can potentially reduce the number of tests performed and speed up the availability of viral load results, according to a CDC analysis presented at the HIV Diagnostics Conference in March.
Read More »Activated or inactivated? Transfusing the right platelets
August 2019—In the blood bank, all platelets in bags appear to be the same, whether resting or activated, and because they look the same, they may be used as if they’re equal. But they are not.
Read More »Ups and downs of bringing in Beaker AP LIS
August 2019—Having an enterprisewide health care platform can put laboratories in a stronger decision-making position for enterprisewide IT, whereas in most other circumstances, “we are relatively isolated,” said Raj C. Dash, MD, in a talk he gave at this year’s Executive War College. Dr. Dash, vice chair of pathology IT at Duke University Medical Center, shared what he called the blessings and curses of his department’s move in 2014 to a lab information system that’s fully integrated with the electronic medical record. His focus was Beaker’s AP-LIS module.
Read More »AMP case report: Coexisting somatic JAK2 V617F pathogenic variant and likely germline calreticulin exon 9 nonpathogenic variant in a patient with newly diagnosed ET
August 2019–Newly discovered pathogenic variants in BCR-ABL1-negative myeloproliferative neoplasms (i.e. polycythemia vera, essential thrombocythemia, primary myelofibrosis) led to recent revisions of the World Health Organization diagnostic criteria. Initially JAK2 V617F, MPL (MPL W515K/L), and calreticulin (CALR) exon 9 gene pathogenic variants were deemed mutually exclusive in patients with essential thrombocythemia and primary myelofibrosis. However, coexisting somatic variants in both JAK2 V617F and CALR have been reported with variable frequency, ranging from less than one percent and up to 6.8 percent depending on the employed molecular technique.
Read More »In one spot: surgical pathology specimen handling specifics
August 2019—A practical guide that can help labs standardize the handling of a patient’s surgical specimen from harvest to diagnosis is available but too little known, and one of its authors aims to change its hidden treasure status. The 52-page “Practical Guide to Specimen Hand-ling in Surgical Pathology” is on the CAP/NSH Histotechnology Committee page on the CAP website. “Our main objective was to standardize specimen collection handling. Nothing had ever been done like it before,” says Elizabeth Sheppard, MBA, HT(ASCP), past president of the National Society for Histotechnology and head of global market access at Roche Tissue Diagnostics, Tucson, Ariz. She and M. Elizabeth H. Hammond, MD, first chair of the CAP Center Guideline Committee, submitted the topic for an evidence-based guideline; however, it was determined to be better suited as a practical guide for labs to be developed by the CAP/NSH Histotechnology Committee.
Read More »Cytopathology in focus: Lab performance in 2018—a year-end tally
August 2019—The CAP has a long-standing commitment to education in cytopathology, with a number of organized educational offerings in gynecologic and nongynecologic cytopathology. The Interlaboratory Comparison Program in nongynecologic cytopathology (NGC Education) was started in 1997 and the Interlaboratory Comparison Program in fine-needle aspiration glass slide education (FNAG) in 2010. These programs are strictly educational and not graded or used for proficiency testing. Semiannual (FNAG) and quarterly (NGC) mailings include four or five cases. For each case, glass slides generally stained with a Diff-Quik and/or Pap stain are provided.
Read More »Cytopathology in focus: BD Onclarity HPV assay now in CAP HPV Surveys
August 2019—The BD Onclarity HPV assay is a human papillomavirus test approved by the Food and Drug Administration on Feb. 12, 2018. The assay is a qualitative test for detection of HPV in cervical specimens collected either with a broom or endocervical brush/spatula combination and placed in a BD SurePath liquid-based cytology vial. The assay is not approved for use with ThinPrep collection media (PreservCyt).
Read More »Cytopathology in focus: Master’s for all—unifying training in cytotechnology
August 2019—Cytology practice is shifting from fewer gynecologic screening tests to a greater focus on diagnostic testing in nongynecologic cytology including fine-needle aspiration. Roles of cytotechnologists have been changing in the workplace to help laboratories meet new demands and act as pathologist extenders.
Read More »Put It on the Board
A heads-up on hybrid lab models of genetic testing
August 2019—Little attention has been paid to the emergence and effect of hybrid laboratories—those that fall in the middle ground between the direct-to-consumer and traditional models, say the authors of an opinion piece published in the June 25 issue of JAMA. Kathryn Phillips, PhD, Julia Trosman, PhD, and Michael Douglas, MS, of the Center for Translational and Policy Research on Personalized Medicine, University of California, San Francisco, say the emergence of the hybrid model, in which a clinician orders the test and returns results, “has significant implications for everyone involved in genetic testing,” providing potential benefits and risks.Greater access and convenience and lower cost for the consumer are among the potential benefits they list. Among the concerns: reduced continuity of care, profit motivation, and insufficiently extensive guidance and counseling. But there are other challenges with hybrid laboratories, they say.
Q&A column
Q. I have heard that in the United States there is a shortage of tuberculin skin test antigen used for detecting Mycobacterium tuberculosis infection. Is there an alternative that can be used? Read answer. Q. What is the value of doing incubated mixing studies on prolonged prothrombin times? Read answer.
Read More »Clinical pathology selected abstracts
Editor: Deborah Sesok-Pizzini, MD, MBA, professor, Department of Clinical Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, and chief, Division of Transfusion Medicine, Children’s Hospital of Philadelphia. Financial impact of approaches to reduce bacterial contamination of platelet transfusions August 2019—The leading infection risk from blood transfusion is bacterial contamination, which is most likely due to the ...
Read More »Anatomic pathology clinical abstracts
Evaluation of pan-TRK IHC in infantile fibrosarcoma, lipofibromatosis-like neural tumor, and histological mimics.
August 2019—Infantile fibrosarcoma is characterized by intersecting fascicles of spindle cells and ETV6-NTRK3 gene fusion in most cases. Given histological overlap with other spindle-cell tumors, the diagnosis can be challenging and often requires molecular confirmation. A recently developed pan-TRK antibody shows promise for identifying tumors with NTRK fusions.
Newsbytes
August 2019—From concept to curriculum: PIER going strong five years later: In the five years since its launch, Pathology Informatics Essentials for Residents, or PIER, has continued to serve as a much-needed guide for pathology residents and program directors who otherwise would be navigating the waters of informatics training without a compass.
Read More »From the President’s Desk—QI: rigor, precision, scope, and depth
August 2019—I am always interested in learning what is new in CAP laboratory improvement. My first inspection was some 40 years ago and our work in that realm has grown faster and gone further than I could have imagined back then.The CAP accredited its first laboratory in 1964 and published the first checklist several years later. I’m told that when Dennis B. Dorsey, MD (a then-future CAP president), submitted the first draft at 10 pages, his fellow LAP commissioners asked that he try to make it shorter.
Read More »Molecular pathology selected abstracts
Editors: Donna E. Hansel, MD, PhD, chair of pathology, Oregon Health and Science University, Portland; Richard D. Press, MD, PhD, professor and director of molecular pathology, OHSU; James Solomon, MD, PhD, assistant professor, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine, New York; Sounak Gupta, MBBS, PhD, senior associate consultant, Mayo Clinic, Rochester, Minn.; Tauangtham Anekpuritanang, MD, molecular pathology ...
Read More »TDM to the rescue in biologics boom
July 2019—In the early, heady days of biologic therapies, use of these drugs resembled a common military tactic of the Civil War: charge and retreat, charge and retreat, charge and retreat. The approach, though modern at the time, often proved disastrous. Jeffry Katz, MD, recalls the excitement that greeted the arrival of anti-tumor necrosis factor-α agents, starting with infliximab (Remicade) in 1998. “What we used to do is we would give patients a drug, and we would wait for them to get sicker,” says Dr. Katz, medical director, inflammatory bowel diseases, University Hospitals Cleveland Medical Center, and professor of medicine, Case Western Reserve University School of Medicine.
Read More »Idea to ease PT standard for HbA1c stirs alarm
July 2019—Excellence is considered the hallmark of progress throughout health care. We don’t expect to hear that, for a particular diagnostic test, standards may already be too high.
Read More »A killer app comes out of the crowd
July 2019—Twenty years ago, Ulysses Balis, MD, bought the domain name HistoQuery.org. He had realized that one day, digital pathology would be sophisticated enough to be incorporated into interactive, Web-based tools, so he did what any self-styled geek would do with such a nascent idea—he waited.
Read More »Close-up of HIV-2 qualitative RNA and viral load testing
July 2019—Qualitative HIV-2 RNA testing to resolve discordant HIV-2 results may be warranted but seldom results in confirmation of HIV-2 infection, Linda M. Styer, PhD, illustrated with data at the 2019 HIV Diagnostics Conference in March.
Read More »Letters
July 2019—We thoroughly enjoyed the article by Anne Paxton, “Microscopy’s dangers: From wear and tear to disabling injury” (April 2019). This is an underrepresented topic in the pathology world. I have been practicing for nearly 11 years; we are a group of 13 pathologists including part-time and full-time pathologists. Two female senior pathologists in our group underwent neck surgery less than a year apart owing to years of accumulated trauma.
Read More »In memoriam
July 2019—Elizabeth R. Cary, MD, state commissioner for Mississippi for the CAP Laboratory Accreditation Program, died on May 31 at age 81.Dr. Cary was the chief of clinical pathology at the G.V. (Sonny) Montgomery VA Medical Center in Jackson, Miss. In 1994, the CAP appointed Dr. Cary as Mississippi state commissioner. The following year, the VA appointed her as regional commissioner for the national VA system.
Read More »Hemostasis testing: What is the impact of direct oral anticoagulants?
July 2019—Prevention and treatment of venous thromboembolic disease is accomplished through the use of anticoagulant agents, which are prescribed for millions of Americans annually. A revolution in anticoagulant use has occurred over the last decade, as direct oral anticoagulants (DOACs) were introduced to the market. The new agents have a number of advantages over warfarin, the traditionally administered oral agent, which is a vitamin K antagonist (VKA).
Read More »From CAP Press: In new book, a practical approach to renal biopsy
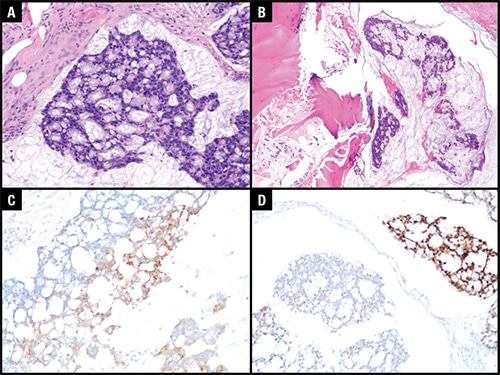
July 2019—New from CAP Press is Medical Kidney Diseases—Morphology-Based Novel Approach to Renal Biopsy, by Huma Fatima, MD, assistant professor and director of the renal pathology laboratory, Department of Pathology, University of Alabama at Birmingham. It presents a simple and practical approach to renal biopsy by providing a pertinent differential diagnosis related to various patterns of injury involving renal parenchyma by light microscopy and reaching a correct diagnosis by assimilating immunofluorescence and electron microscopy findings. The 90-page book contains 66 cases, two of which we are reprinting here.
Read More »Puzzles, pearls: diagnosing interstitial lung disease
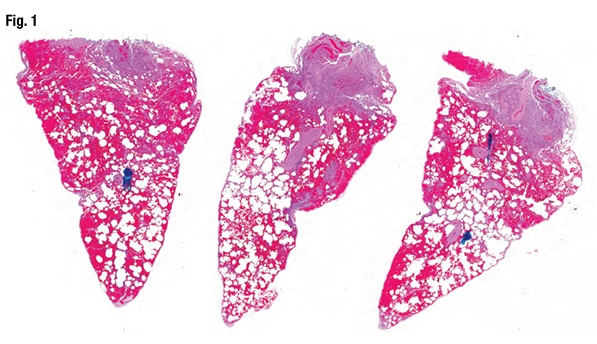
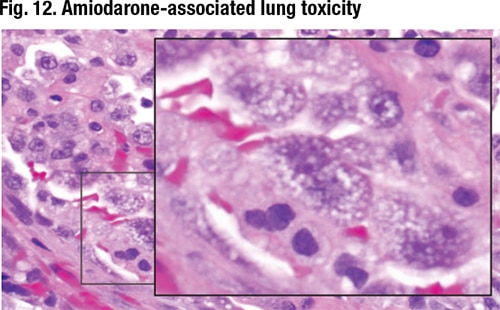
July 2019—Most fresh blood in lung wedge biopsies is artifact, but when it’s diffuse alveolar hemorrhage, the pathologist must call the clinician because DAH patients can go downhill fast. Maxwell L. Smith, MD, a consultant in the Department of Laboratory Medicine and Pathology at Mayo Clinic Arizona and associate professor, Mayo Clinic School of Medicine, shared that pearl from one of the 10 consultation cases he and Brandon T. Larsen, MD, PhD, co-presented in their CAP18 session on diagnosing interstitial lung disease. Their discussion of two of those cases follows.
Read More »Chemistry and immunoassay testing: Standardizing platforms, ranges, interfaces—panel weighs in
July 2019—One vendor or two. Automating esoteric testing. The desire for more smart systems. The need for analytics. Seven people spoke with CAP TODAY publisher Bob McGonnagle in May about chemistry and immunoassay testing. They are David Alter, MD, DABCC, of Emory University; Nina Babic, PhD, DABCC, of Medical University of South Carolina; Denise Pastore of Siemens Healthineers; Timothy Lenz, PhD, of Randox; Delena Carite of Roche Diagnostics; and Jessica Tubman, MPH, MT(ASCP), and Stephen Ishii, MT(ASCP), of Beckman Coulter. What follows is what they told us.
Read More »Put It on the Board
AMP bolsters position on consumer genomic testing
July 2019—The Association for Molecular Pathology revised its position for all consumer genomic testing. Based on a recent assessment of the current market landscape and privacy best practices, the latest position statement features an expanded list of conditions that must be met before the AMP can support a clinically meaningful test. The AMP remains neutral to all recreational, novelty, and ancestry testing that may create educational opportunities for the public. The expanded set of conditions is as follows: • All health-related claims must have well-established clinical validity. • The consumer genomic testing provider must comply with the CLIA statute and regulations. Test validation and interpretation should be performed by board-certified molecular laboratory professionals. • Information regarding the analytical and clinical validity of the tests should be present in all marketing materials and included in each report of results.
Q&A column
Q. What is the current standard practice for collection tube order for CSF testing? Read answer. Q. What is the significance of the absence of coagulation of seminal fluid in a patient who previously experienced normal seminal fluid coagulation, followed by normal liquefaction, and had fathered children? Are there medications that can prevent seminal fluid coagulation? Read answer.
Read More »Newsbytes
Digital pathology: from education to implementation July 2019—Matthew Hanna, MD, is well aware that not all pathologists embrace the idea of using digital pathology for clinical applications. “I’m very confident it’s a familiarity issue,” says Dr. Hanna, clinical instructor in breast pathology and informatics at Memorial Sloan Kettering Cancer Center.
Read More »From the President’s Desk: CAP19—Widening our embrace
July 2019—I attended our first CAP annual meeting and haven’t missed one since. Pathology is dynamic and complex, so our learning team is forever coming up with useful and meaningful ways to present new material. Everyone who is involved in any way wants to make our time in Orlando engaging and enjoyable. CAP19 will be the best ever.
Read More »Clinical pathology selected abstracts
July 2019—Noninvasive testing for prenatal screening has been available through independent commercial companies and academic and hospital-based laboratories since 2011.
Read More »Anatomic pathology selected abstracts
July 2019—Although the majority of low-grade, early stage endometrial cancer patients have good survival rates with surgery alone, patients who recur tend to do poorly. Identifying patients at high risk of recurrence who would benefit from adjuvant treatment or more extensive surgical staging would improve individualized care for endometrial cancer patients.
Read More »Molecular pathology selected abstracts
July 2018—The use of liquid biopsies to non-invasively detect and monitor cancer is rapidly expanding. In these assays, fragments of cell-free DNA (cfDNA), which are released when cells undergo necrosis or apoptosis, are isolated from the patient’s plasma and sequenced. Because cfDNA can originate from cancer cells and normal cells, the variant allele frequency of cancer-specific somatic mutations can often be very low.
Read More »Genetics lands in primary care inboxes
June 2019—It took David Ledbetter, PhD, a mere six years or so to become a hero. Dr. Ledbetter, executive vice president and chief scientific officer, Geisinger, had helped oversee the expansion of the health system’s MyCode precision medicine project, which began as a traditional research biobank in 2007.
Read More »ME multiplex panel: debating the tradeoffs
June 2019—Meningitis and encephalitis have been called by some the most terrifying diseases in medicine, in part because of the difficulty of diagnosing their underlying pathology. The clinical stakes of laboratory testing are high for diagnosing and treating the inflammation that meningitis/encephalitis (ME) causes within the central nervous system.
Read More »Sidestepping pitfalls in diagnosing interstitial lung disease
June 2019—The pathologic approach to evaluating specimens as part of a workup for medical lung disease demands a different strategy than is typically used for the patient with a question of neoplasia, says Brandon T. Larsen, MD, PhD.
Read More »Path to importance of PD-L1 status in breast cancer
June 2019—New data support testing patients for their PD-L1 immune cell status when they are diagnosed with metastatic or unresectable locally advanced triple-negative breast cancer to determine if they might benefit from a checkpoint inhibitor.
Read More »Earlier HIV detection with prototype Abbott assay
June 2019—Abbott unveiled a new and improved fourth-generation prototype HIV assay at the 2019 HIV Diagnostics Conference in March. In an Abbott-funded study, the prototype assay was compared with the fourth-generation Abbott Architect HIV Ag/Ab Combo and Roche Elecsys HIV Combi PT run on the Cobas e602.
Read More »Bladder cancer detection and surveillance: How urine cell-free DNA stacks up against cytology
June 2019—A high-throughput sequencing panel was found to be more than 90 percent sensitive in detecting urinary tumor DNA in early-stage bladder cancer and in post-treatment surveillance. The approach, reported in April in Cancer Discovery, overcomes some of the challenges urinary cell-free DNA analysis poses, said one of its developers, and is far more sensitive than cytology and cystoscopy.
Read More »Talking tests, instruments, and what’s best where
June 2019—Analyzers, menus, test distribution, and middleware were the topics of a roundtable led in April by CAP TODAY publisher Bob McGonnagle. Samuel McCash, MD, Frederick Kiechle, MD, PhD, Christina Reita of Roche, and Mimi Dang of Tosoh talked IT, turnaround time, and what challenges stand out. Here is what they told us.
Read More »Put It on the Board
Minimum set of alleles recommended for clinical CYP2C9 genotyping
June 2019—A joint report from the CAP and the Association for Molecular Pathology was published last month to aid in the design and validation of clinical CYP2C9 assays, promote standardization of testing across different laboratories, and improve patient care. The report, “Recommendations for Clinical CYP2C9 Genotyping Allele Selection: A Joint Recommendation of the Association for Molecular Pathology and College of American Pathologists,” was released online ahead of publication in the Journal of Molecular Diagnostics. The AMP Pharmacogenetics Working Group is developing a series of guidelines to help standardize clinical testing for frequently used genotyping assays. Developed with organizational representation from the CAP and the Clinical Pharmacogenetics Implementation Consortium, the latest report follows a set of recommendations for clinical CYP2C19 genotyping allele selection published in May 2018.
Q&A column
Q. Is there a specific CAP recommendation regarding which anticoagulants are acceptable for synovial fluid crystal analysis? If not, what does the CAP recommend? Read answer. Q. What is the next step in resolving platelet clumping when it also occurs in a citrate tube? Read answer.
Read More »Newsbytes
June 2019—How Orchard Software is helping labs address transgender care: Establishing useful reference ranges for cisgender patients can be difficult, and for transgender patients it can be even more challenging. Add to this the desire to show the reference ranges for transgender patients as separate categories, distinct from the values for male and female patients, and the challenges mount for some medical centers. Read more.
Read More »From the President’s Desk: Best-kept secret in medicine
June 2019—I wasn’t one of those kids who always knew they wanted to be a doctor. Science was a powerful draw, which might have suggested medical school if my sister hadn’t gotten there first. But she did, so I majored in chemistry at Vanderbilt. As an undergraduate with little money, I hoped to fast-track, so I found a summer job anesthetizing laboratory rats in the middle of the night and removing their kidneys. The work supported a group studying the renin-angiotensin system. They thought I had a knack for surgery, which prompted a reassessment of my chemistry major and eventually led to an application to medical school. Life makes choices for us sometimes, and I was lucky that way.
Read More »Clinical pathology selected abstracts
June 2019—Dietary patterns during adulthood and cognitive performance in midlife. Cognitive impairment is associated with an increased risk of mortality, disability, and late-life dementia, which contributes to the rising costs of health care. Several studies have demonstrated cognitive decline in midlife, and some data have linked this decline to cardiovascular disease risk factors or a more sedentary lifestyle. Diet is a modifiable exposure, but few studies have analyzed the risk of cognitive impairment due to dietary factors.
Read More »Anatomic pathology selected abstracts
June 2019—Markers for differentiating triple-negative breast cancer from TTF1-negative lung adenocarcinoma. Triple-negative breast cancer patients have an increased risk of developing visceral metastases and other primary nonbreast cancers, particularly lung cancer. The differential diagnosis of triple-negative breast cancer (TNBC) metastases and primary cancers from other organs can be difficult due to lack of a TNBC standard immunoprofile.
Read More »Molecular pathology selected abstracts
June 2019—Link between immunogenic neoantigens derived from gene fusions and T-cell responses. Immunotherapy is quickly emerging as an important therapeutic strategy for hematologic and solid tumors. In principle, immune cells recognize unique non-self antigens expressed by cancer cells and this serves as the initial step in eliminating such cells. Among the metrics used to assess the likelihood of response to immunotherapy is the presence of a high level of somatic mutations, referred to as tumor mutation burden (TMB). This serves as a surrogate for extrapolating the level of neoantigens ex- pressed by cancer cells.
Read More »Low and inside: reducing staff turnover
May 2019—When Monica Rocheford and colleagues at Allina Health Laboratory first began digging into rising turnover rates at various locales within the system, the effort carried a whiff of concern, if not urgency. One hospital site had jumped from a 10.8 percent turnover rate in 2016 to 44.9 percent two years later. At another site, turnover reached 49 percent in 2018, from 24 percent the year before. The culprit appeared to be a three-letter word: pay. “That was the main reason they were giving us for their resignation,” says Rocheford, system director, laboratory operations, recalling the exit interviews with departing staff. So in 2018, Allina, with nearly 1,000 lab employees (spread across 12 hospitals, a core lab, and roughly 60 clinics in Minnesota and western Wisconsin), awarded a technical increase across the board.
Read More »CBD flies off shelves, fosters uncertainty in tox lab
May 2019—If a futurist had forecast 10 years ago that, in 2019, a compound of the cannabis plant would be sold over the counter and online and consumed for health reasons by an estimated quarter of the U.S. adult population, most people would have scoffed at the suggestion.
Read More »IGHV gene mutation at heart of CLL treatment
May 2019—Chronic lymphocytic leukemia is a neoplasm of small mature B-cells and the most common leukemia diagnosed in adults. Median age of diagnosis is 70 years, but there is a surprisingly large percentage of patients, about 10 percent, who are younger than 55, and it’s not uncommon now to occasionally see CLL patients, about two percent, in their 40s.
Read More »What UCLA learned in seven years of exome sequencing
May 2019—Never go it alone without the input of the ordering clinician, and the diagnostic yield is better than expected. Those are two of the five lessons UCLA learned in its first seven years of clinical whole exome sequencing.
Read More »Mass casualty plan puts point-of-care testing in the ED
May 2019—If a mass casualty event brings patients to Le Bonheur Children’s Hospital in Memphis, Tenn., clinical laboratory staff will head straight to the bedside. Le Bonheur Children’s Hospital is a level-one trauma center. Its new mass casualty response plan, two years in the making, has laboratory staff in the emergency department and triage areas, where they will perform point-of-care testing for frontline providers.
Read More »From CAP Press: A renewed perspective on laboratory administration
May 2019—CAP Press released this month its second edition of Laboratory Administration for Pathologists, first published in 2011. It covers management of personnel, laboratory space, pathology information systems, and quality in laboratory medicine and in the anatomic pathology lab. That’s just to start. Among its other chapters: patient safety, the pathology position, lab laws and regulations, legal affairs, ethics, and financial management of the lab and of the pathology practice. And there is more in the 296-page book edited by Elizabeth A. Wagar, MD, Michael B. Cohen, MD, Donald S. Karcher, MD, and Gene P. Siegal, MD, PhD. CAP TODAY recently asked Dr. Wagar about the latest edition; what she told us appears here, along with an excerpt (at right). Dr. Wagar is professor and chair, Department of Laboratory Medicine, University of Texas MD Anderson Cancer Center.
Read More »New Q-Probes on expression rates in invasive breast cancer
May 2019—A new CAP Q-Probes benchmarking study on expression rates in invasive breast carcinoma will be available beginning in June.
Read More »AMP case report: Response to second-line osimertinib in primary EGFR p.T790M mutation
May 2019—Non-small cell lung cancer patients with epidermal growth factor receptor activating mutations have excellent response to oral therapy with EGFR tyrosine kinase inhibitors. However, development of resistance to first- and second-generation TKIs is a well-recognized phenomenon with acquired p.T790M mutation and accounts for most TKI drug resistance. Resistance to EGFR TKI therapy has been described in tumors with coexistent primary p.T790M mutation and an EGFR activating mutation in a small number of patients.
Read More »In next-gen sequencing, ‘a lot more room to grow’
May 2019—Workflow, data interpretation, communication, and community—that and more came up when CAP TODAY publisher Bob McGonnagle spoke with five NGS experts in April: Boaz Kurtis, MD; Zhiyv (Neal) Niu, PhD; David Eberhard, MD, PhD; Luca Quagliata, PhD; and Arnaud Papin, MSc, MBA. What they said follows.
Read More »Cytopathology in focus: Updated NSCLC guideline moves molecular cytopathology forward
May 2019—The genomic landscape of non-small cell lung carcinoma is evolving constantly with the discovery of a growing number of molecular alterations and associated targeted therapies that have an impact on patient care. The CAP, International Association for the Study of Lung Cancer, and Association for Molecular Pathology issued a guideline in 2013 to provide a road map for molecular testing to select patients for treatment with targeted tyrosine kinase inhibitors.
Read More »Cytopathology in focus: What pathologist competencies are monitored and how
May 2019—The CAP regularly surveys the practices of the laboratories participating in the CAP Nongynecologic Cytopathology Education Program, or NGC. Members and staff of the CAP Cytopathology Committee developed a supplemental questionnaire eliciting feedback on pathologist competency activities. The Survey was mailed to 2,142 participants in the NGC-B 2018 education program. The pathologist competencies queried were as follows:
Read More »Cytopathology in focus: Exchange of views—HPV screening policies in Australia
May 2019—In the November-December 2018 issue of the Journal of the American Society of Cytopathology is a fascinating analysis of human papillomavirus screening policies in Australia by researchers from New Zealand, a rebuttal by members of an Australian Cervical Cancer Screening Guidelines Working Party, and a thoughtful editorial by cervical cancer screening experts from the United States and England.
Read More »Put It on the Board
Early Sepsis Indicator receives 510(k) clearance
May 2019—Beckman Coulter’s Early Sepsis Indicator received 510(k) clearance from the Food and Drug Administration. Beckman Coulter says clinical trial findings showed that its monocyte distribution width biomarker best discriminated sepsis from all other conditions when combined with the current standard of care. The Early Sepsis Indicator is automatically reported as part of a routine complete blood count with differential for adult emergency department patients. A positive Early Sepsis Indicator result signals a higher probability of sepsis. Compared with reviewing WBC count alone, Beckman Coulter says the Early Sepsis Indicator strengthens a clinician’s suspicion of sepsis by 43 percent and, together with clinical signs and symptoms, improves confidence in helping to rule out sepsis by 63 percent. The indicator can be used in conjunction with the company’s Multidiscipline Reflex Rules in Remisol Advance middleware.
Q&A column
Q. Are there any FDA-approved laboratory-developed assays or point-of-care assays for detecting oxycodone or fentanyl in urine or blood? Read answer. Q. What was the cause of a sudden shift in values in the triglyceride assay at one clinical laboratory? Read answer.
Read More »Newsbytes
May 2019—Excel tool color codes persistent problems in lab test ordering: Variety may be the spice of life, but in the pathology lab, it may present a conundrum. Case in point: The LIS is a rich source of data about lab test utilization, but managing millions of disparate pieces of data to assess test utilization can be difficult. But not to Tylis Y. Chang, MD, of Northwell Health.
Read More »From the President’s Desk—CAP Center: If we couldn’t do it, who could?
May 2019—The CAP Center for Pathology and Laboratory Quality for Evidence-Based Guidelines has partnered or collaborated with more than 20 societies to produce 15 published laboratory practice guidelines—with 10 more underway. The Center is one of the best things we do and one of the things we do best.One thing about the CAP: We make no small plans. It was that way when we decided to inspect and then accredit laboratories. It was that way when we decided to hold an independent annual meeting. And it was that way when the CAP Center had its first official guidelines published in Archives of Pathology & Laboratory Medicine.
Read More »Clinical pathology selected abstracts
May 2019—Optimization of laboratory ordering practices for CBC with differential: Over 5 billion laboratory tests are performed in the United States each year, and more than 20 percent are considered unnecessary. The American Board of Internal Medicine Foundation initiated the Choosing Wisely campaign in 2012 to increase awareness of wasteful or unnecessary medical tests, procedures, and treatments. Studies have shown that tests ordered without a clear rationale not only waste resources but are also a source of iatrogenic anemia, which has been associated with increased blood transfusions, lengths of stay, and mortality.
Read More »Anatomic pathology selected abstracts
May 2019—ALK-rearranged tumors in the STUMP subcategory of uterine tumors: Smooth muscle tumor of uncertain malignant potential is a rare diagnosis rendered when there is uncertainty concerning the biological potential of a smooth muscle tumor. The initial differential diagnosis is often broad, as tumors in this subgroup are morphologically heterogeneous. Recent data suggest that uterine inflammatory myofibroblastic tumors (IMTs) with anaplastic lymphoma kinase (ALK) rearrangement may be misclassified as smooth muscle tumor of uncertain malignant potential (STUMP), but the extent to which this occurs has not been examined.
Read More »Molecular pathology selected abstracts
May 2019—Diagnosing and targeting pancreatic cancer at the single-cell level: Pancreatic ductal adenocarcinoma is a highly aggressive cancer that is often unresectable at the time of diagnosis. It also is frequently metastatic and associated with a high mortality rate. One approach to reduce the poor outcomes associated with this disease could involve early detection and treatment of the cancer before it develops the ability to invade and spread to other organs.
Read More »Next act in genomics: the consumer orders
April 2019—For years, laboratories have chafed against testing being, literally and figuratively, an out-of-sight, out-of-mind transaction. Now a new, highly visible era in genetics may be pushing testing the other way, into the hands of consumers who value entertainment as well as medical information. Anyone who wants to write a book about this shift has a ready-made title: From Basement to Big Top. It’s not that clinical testing is becoming an actual circus. But ever since the first consumer genetic tests entered the market in 2007—in a nonphysician-ordered, SNP array technology way—labs, physicians, and regulatory agencies have had plenty to juggle.
Read More »Microscopy’s dangers: From wear and tear to disabling injury
April 2019—When pathologist Sandra Ewaskow, MD, was asked at a recent medical conference what topic she would choose if she were to write a book in her field, she thought of her own experience with musculoskeletal pain and of her mother, who had recently been hospitalized for occupational therapy after a hip fracture.
Read More »Study gauges impact of genotyping on gonorrhea treatment
April 2019—Genotypic testing for ciprofloxacin susceptibility in Neisseria gonorrhoeae has been proved to be effective in guiding physician treatment in a single-center study at UCLA Health.
Read More »Too few technologists: labs take inventive steps
April 2019—The tight supply of technologists to fill open positions is pushing laboratories to be creative in finding answers. Memorial Sloan Kettering Cancer Center and TriCore Reference Laboratories found their answers by looking not just outward but also—and largely—inward.
Read More »Quantitative image analysis: In guideline, preliminary rules for pathology’s third revolution
April 2019—With the release in January of a new guideline for quantitative image analysis of HER2 immunohistochemistry for breast cancer, the CAP believes it is filling a gap and blazing a trail for the profession. In setting evidence-based standards, the guideline provides background and details about the quantitative image analysis (QIA) process and the data and metadata it generates. The guideline will help facilitate pathology’s increasing use of not only digital pathology but also artificial intelligence, says Marilyn Bui, MD, PhD, chair of the CAP expert panel for QIA of HER2 IHC. “This is not just another guideline. It is a milestone for pathologists.”
Read More »In memoriam: Harold E. Bowman, MD (1925–2019)
April 2019—Harold E. Bowman, MD, a member of the CAP Board of Governors from 1979 to 1985, died on Feb. 1 at age 93. Dr. Bowman retired in 1994 as director of laboratories at St. Lawrence Hospital, now Sparrow Hospital, in Lansing, Mich., and as associate chair, Department of Pathology, Michigan State University College of Human Medicine.
Read More »AMP case report: Acute promyelocytic leukemia with cryptic t(15;17) identified by RT-PCR
April 2019—Acute promyelocytic leukemia (APL) is a subtype of acute myeloid leukemia (AML) in which promyelocytes predominate. APL accounts for about 10 percent of AML cases, and although APL can be diagnosed at any age, it is most common among young adults with a slight male predominance. APL is defined by the balanced reciprocal translocation (15;17)(q22;q21) between PML and RARA, although variant translocations involving RARA and other partner genes can occur.
Read More »Peripheral blood evaluation: B12 deficiency, anemia, ET, CMML
April 2019—Diagnoses of essential thrombocythemia and chronic myelomonocytic leukemia were among those covered in four cases in a CAP18 session on practical challenges in peripheral blood evaluation.
Read More »Put It on the Board
Shield launches test for antibiotic susceptibility in N. gonorrhoeae April 2019—Shield Diagnostics launched Target-NG, a rapid molecular test for antibiotic susceptibility in Neisseria gonorrhoeae. “Rapid molecular testing for ciprofloxacin resistance allows for smarter medicine,” Jeffrey D. Klausner, MD, MPH, a professor of infectious disease medicine at the University of California, Los Angeles, said in a Shield statement. “Right now we’re treating gonorrhea with a sledgehammer; we’re treating everything with the same exact regimen. And it’s not a surprise that the organism will become resistant to what we’re currently using.” Ciprofloxacin can be used to treat 80 percent of infections and is 99.8 percent effective when susceptibility has been determined. Because it is administered as a single oral dose, rather than the current injectable treatment, clinicians can prescribe antibiotics for the patient to give to their partners. “Shield has launched Target-NG to help clinicians adopt a precision medicine approach to gonorrhea treatment,” said Nidhi Gupta, PhD, lead scientist on the project.
Read More »Q&A column
Q. Can you describe the contemporary significance and use of osmolality testing in the clinical laboratory? Read answer. Q. Is it necessary to include a comment on the patient report indicating that a test result was obtained after dilution? Read answer.
Read More »Newsbytes
April 2019—The R&B classic “Time Is on My Side” may be an anthem for rejected lovers, but a new virtual reality teaching tool that allows students to “visit” the pathology lab without leaving the classroom may soon have NYU medical students humming the song’s refrain.
Read More »From the President’s Desk: CAP Learning: building on feedback
April 2019—As Dr. Seuss famously wrote, “It is fun to have fun, but you have to know how.” CAP Council on Education chair Jennifer Hunt, MD, MEd, agrees. Learning styles evolve as we mature, she says; grownups are not just tall children. We know what we want to do and are drawn to knowledge we can use. Most pathologists are born educators; we can’t help ourselves. We like to think about how we learn. I think that’s why the CAP Learning team does such an outstanding job—they know what to ask, how to listen, and when to act. Participant evaluations are scrutinized and what we learn from them is applied quickly.
Read More »Clinical pathology selected abstracts
April 2019—Trauma resuscitation considerations: gender as a biological variable: Sex dimorphisms in coagulation are well established, with females manifesting a more hypercoagulable profile, but the relationship between sex dimorphism in coagulation and trauma outcomes has not been investigated. Trauma-induced hemorrhage remains a leading cause of early post-injury death.
Read More »Anatomic pathology selected abstracts
April 2019—Frequent GNAQ and GNA14 mutations in hepatic small vessel neoplasm: April 2019—Hepatic small vessel neoplasm is a recently described infiltrative vascular neoplasm of the liver composed of small vessels. Although its infiltrative nature can mimic angiosarcoma, hepatic small vessel neoplasms (HSVNs) are thought to be benign or low-grade neoplasms because they lack cytologic atypia and increased proliferation.
Read More »Molecular pathology selected abstracts
April 2019—Variants in NUDT15: association with thiopurine–induced myelosuppression: The thiopurines mercaptopurine, thioguanine, and azathioprine are purine antimetabolites widely used as anticancer and immunosuppressive agents. Commonly prescribed in patients with inflammatory bowel diseases (IBD), such as Crohn’s disease and ulcerative colitis, they are valuable steroid-sparing treatment options.
Read More »Wading deeper into liquid biopsy
March 2019—The standard riff for talking about a promising new cancer test should be familiar to anyone within sneezing distance of a laboratory: There’s no one-size-fits-all assay. But if any test were to come close, it would be liquid biopsy. Are clinicians eager to use it? Check. Is it relatively simple to do (check) with fairly quick turnaround times (check)? Does it work for solid and hematological tumors? Check and check. Across multiple specimen types—serum, urine, vitreous fluid, cerebrospinal fluid, stool? Quite likely. Can it be used to characterize patients’ molecular profiles, monitor therapy, assess tumor evolution, identify resistance mechanisms, and detect early disease and minimal/measurable residual disease? Half a dozen checks. Even if liquid biopsy does fall short of a one-size-fits-all assay, it’s doing a reasonable impression of a Swiss Army knife (if not Sergeant Troy’s sword fantastic, for those of you who are Thomas Hardy fans).
Read More »FDA nudges standards adoption in electronic reporting
March 2019—An interesting medical informatics moment occurred post-9/11 with the delivery of anthrax-poisoned letters to several congressional and news media offices. “There was not a single anthrax test order communicated to the CDC electronically,” says Steven H. Hinrichs, MD.
Read More »Practical challenges in peripheral blood smear evaluation
March 2019—Unlike high-count monoclonal B-cell lymphocytosis, low-count MBL has a limited chance of progression to CLL and thus there is no need for follow-up. And a definitive diagnosis of T-cell large granulocytic leukemia requires demonstration of clonality.
Read More »Labs add safety net to critical values procedure
March 2019—A hypercritical value notification policy at Northwell Health in New York State authorizes the laboratory alone to activate a hospital’s rapid response team when necessary. This is the newest step in a critical value notification process that had been working well—but not quite well enough.
Read More »Exploring MALDI-TOF mass spec for mycobacteria
March 2019—The family of Mycobacteriaceae includes the genus Mycobacterium, which includes more than 190 species. These organisms have an unusual cell wall. Those of us who work in mycobacteria laboratories know that the cell wall has a high lipid content that makes these organisms acid fast. Thus they’re acid-fast bacteria.
Read More »Can NGS replace routine respiratory testing? Study says not yet
March 2019—A small study performed at the University of Utah found that a next-generation sequencing assay cannot replace routine standard-of-care testing to detect pneumonia in immunocompromised patients and determine their treatment. But it could be ordered when an infection is suspected and all other testing has failed to find the etiology.
Read More »AMP case report: FDA-approved DNA blood test for colorectal cancer prompts patient to undergo colonoscopy
March 2019—Colorectal cancer is the third most diagnosed cancer and the second highest cause of cancer mortality in men and women, and in 2016 it accounted for about nine percent of all diagnosed cancers in the United States. When CRC is detected at an early localized stage, the five-year survival rate is 90 percent. With progression to regional disease, five-year survival remains high, at 71 percent.
Read More »POC testing roundtable: risks, resources, relationships
March 2019—Infection control and the heavy demands on point-of-care coordinators were among the top concerns that came up in a recent CAP TODAY roundtable on point-of-care glucose testing. Publisher Bob McGonnagle spoke with four POC testing experts: Sharon Geaghan, MD, Cynthia Bowman, MD, Steven Cotten, PhD, and Corinne Fantz, PhD. Here is what they told us.
Read More »Semen analysis guide: a benchtop sperm morphology aid
March 2019—CAP Press released late last year its Semen Analysis Benchtop Reference Guide, an illustrated guide with emphasis on sperm morphology. Its 68 laminated pages are divided into sections: specimen collection and macroscopic assessment, sperm count, sperm morphology, and nonsperm cells. Behind the book is the CAP Reproductive Medicine Committee, in particular Erica J. Behnke, PhD, HCLD, of Ohio Fertility Providers, Jacob F. Meyer Jr., PhD, HCLD, of Eastern Virginia Medical School, and Laura L. Nelsen, MD, pathologist and director of cytology at MaineGeneral Medical Center and past chair of and now advisor to the committee.CAP TODAY asked Dr. Nelsen about the benchtop reference guide, sample pages of which appear below. Here is what she told us.
Read More »Put It on the Board
Lumipulse G β-Amyloid Ratio test has breakthrough device designation
March 2019—Fujirebio Diagnostics received on Feb. 1 breakthrough device designation from the FDA Center for Devices and Radiological Health for its Lumipulse G β-Amyloid Ratio (1-42/1-40) quantitative in vitro diagnostic test. The test uses measurable β-amyloid 1-42 and β-amyloid 1-40 concentrations found in human cerebral spinal fluid and combines those concentrations into a numerical ratio of β-amyloid 1-42/β-amyloid 1-40 to estimate the presence of β-amyloid neuritic plaque pathology in the brain. The Lumipulse G β-Amyloid Ratio (1-42/1-40) combines the results of Lumipulse G β-amyloid 1-42 and β-amyloid 1-40 using the Lumipulse G System. The ratio results are intended to aid in assessing adult patients, ages 50 and over, who present with cognitive impairment and are being evaluated for Alzheimer’s disease and other causes of cognitive decline. The results must be interpreted in conjunction with other diagnostic tools such as neurological examination.
Q&A column
Q. I just read the 2018 HER2 guideline update. Can you provide an example of how a previously equivocal case is resolved under the new guideline? Read answer.
Read More »Newsbytes
Building a lab or modernizing? Don’t forget the following March 2019—Building a new pathology lab or revamping an existing one gives laboratory decision-makers an opportunity to rethink information technology infrastructure and address persistent problems, plan for new technology, and improve processes.
Read More »From the President’s Desk: Our study of member services and support
March 2019—We launched the CAP member services and support strategy two years ago, setting out to figure out which benefits were most valued by the greatest number of members, identify places where we could find better ways to direct or maintain them, and see where we could be falling short. To keep everyone connected and everything on track, we created a coordinating group whose members had access to a fine staff and thoughtfully curated findings from years of member surveys and market research. Our own surveys showed how the interests and needs of our members overlapped. The market provided context.
Read More »Clinical pathology selected abstracts
March 2019—Evaluation of ethanol interference on routine biochemical tests: Ethanol, a central nervous system depressant widely consumed in many societies, is metabolized in the liver through multiple enzymatic pathways. If the liver’s capacity is exceeded, the excess alcohol will flood into the systemic circulation.
Read More »Anatomic pathology selected abstracts
March 2019—ALK expression in angiomatoid fibrous histiocytoma: a potential diagnostic pitfall: The authors recently encountered a case of primary pulmonary angiomatoid fibrous histiocytoma (AFH), which was initially misdiagnosed as inflammatory myofibroblastic tumor based in part on anaplastic lymphoma kinase expression by IHC. Prompted by this experience, they evaluated anaplastic lymphoma kinase (ALK) expression in 11 AFH, 15 inflammatory myofibroblastic tumors (IMT), and 11 follicular dendritic cell sarcomas using three antibody clones: D5F3, 5A4, and ALK1.
Read More »Molecular pathology selected abstracts
March 2019—Using circulating cell-free fetal DNA to test for monogenic disorders: Screening for fetal chromosomal abnormalities can be performed by noninvasive methods in which fetal cell-free DNA (cfDNA) circulating in the maternal blood is isolated and analyzed. However, the standard of care for screening for monogenic diseases remains population-based carrier screening—testing the parents for their carrier status of deleterious genes.
Read More »Digital pathology matchmaking: people, pixels
February 2019—Digital pathology is many things. One thing it’s not is a one-night stand. As laboratories contemplate using digital pathology for primary diagnosis in the wake of the FDA’s approval nearly two years ago, it’s become abundantly clear that while digital pathology might seem to promise easy pleasure, it’s actually as complicated as keeping multiple spouses happy. Think Jacob, Rachel, and Leah. Think “Big Love.”
Read More »HbA1c platforms studied for lipemia interference
February 2019—A forgotten creditor. A poor relation. An envious rival. In the theater, one of these characters often emerges from the woodwork, ready to supply a plot twist just when the protagonist is riding highest.
Read More »Apocrine breast cancer, ESR1 mutations at center of tumor board review
February 2019—Two breast cases—one of apocrine carcinoma and androgen receptor overexpression and another of metastatic ER-positive cancer and an ESR1 mutation—were the focus of a molecular oncology tumor board session at CAP18.
Read More »Conference shines light on latest in mass spec
February 2019—Mass spectrometry has been proved to be an essential and powerful platform for clinical diagnostics. The value it adds to health care has increased steadily in the past decade.
Read More »Know them when you see them: parasites in tissue
February 2019—In one case, Dr. Ribes found herself intrigued by structures in a patient’s urine cytology specimen. A 36-year-old man from the Republic of Congo had developed dysuria, increased urinary frequency, and terminal hematuria. What they found in the Pap stain in cytology, she said, were large purple structures with the knob at the end.
Read More »AMP case report: Diagnostic pitfalls of testing rare molecular aberrations in lung adenocarcinomas
February 2019—Lung cancer is the second most commonly diagnosed malignancy and results in the most cancer-related deaths each year in the United States, but actionable aberrations in EGFR, ALK, ROS1, and other oncogenes are improving outcomes for a subset of patients.
Read More »Not a fan of training new hires? Lab finds a fix
February 2019—When Tania Hong, BS, MT, joined the University of Vermont Medical Center five years ago, as network director of operations for pathology and laboratory medicine, she interviewed all of the supervisors in her laboratory. She asked what they saw as their biggest challenges.
Read More »AP-LIS vendors on AI, practice complexity, the cloud
February 2019—Practice consolidation and complexity, Web-based and cloud computing, and artificial intelligence. That and more is what writer Valerie Neff Newitt asked six AP-LIS companies about recently. Here is what they had to say. The profiles of their AP information systems are in the Anatomic pathology product guide, along with those of 16 other companies.
Read More »Standard provides labs with predefined LOINC codes for IVD tests
February 2019—Logical Observation Identifiers Names and Codes, or LOINC, is a universal code system for identifying laboratory and clinical observations. LOINC provides unique numeric codes that identify the type of in vitro diagnostic test with defined attributes including component, property, time, system, scale, and method.
Read More »No diagnostics, no stopping antibiotic misuse
February 2017—In a context where the lack of drugs against resistant bacterial pathogens will continue and antimicrobial prescriptions are highly complex, effective antibiotic stewardship programs are strongly needed. In the U.S., the CDC and Centers for Medicare and Medicaid Services highly recommend, while the Joint Commission requires, that all hospitals and nursing care centers have antimicrobial stewardship programs, effective January 2017.
Read More »Put It on the Board
Hologic assay is first FDA-cleared test to detect M. genitalium
February 2019—The Food and Drug Administration granted clearance for Hologic’s Aptima Mycoplasma genitalium assay. This is the first test the FDA has authorized for M. genitalium detection. The FDA reviewed data from a clinical study that included testing of 11,774 samples. The FDA says the study showed that the Aptima assay correctly identified M. genitalium in approximately 90 percent of vaginal, male urethral, male urine, and penile samples. It correctly identified M. genitalium in female urine and endocervical samples 77.8 percent of the time and 81.5 percent of the time, respectively. Vaginal swabs are the preferred sample type owing to better clinical performance. Alternative sample types, such as urine, can be used if vaginal swabs are not available. In addition, the study showed that the test correctly identified samples that did not have M. genitalium present 97.8 to 99.6 percent of the time. The FDA reviewed the Aptima M. genitalium assay through the de novo premarket pathway.
Q&A column
Q. When performing a manual differential that contains immature cells, such as metamyelocytes and myelocytes, do you report an absolute count on all of the individual cells in the myelocytic line, or do you group them together and calculate one ANC? What about lymphocytes and reactive lymphocytes? Read answer. Q. Why and in what employment screening settings is the two-step skin test for Mycobacterium tuberculosis recommended? Read answer.
Read More »From the President’s Desk: Giving group practices what they need most
February 2019—In last month’s column, we talked about practice engagement as an umbrella term for laboratory medical direction and practice management that builds strong relationships within and beyond the laboratory. The CAP Practice Management Committee has been taking the lead on this, but it cuts across multiple domains; the conundrum, as always, is the complexity of what we do. Practice management tools designed for other settings cannot meet our needs because we must address economy, efficiency, effectiveness, and collegiality concerns specific to pathology. But then, affinity for complexity is how we landed here in the first place, so that plays to our strengths.
Read More »Clinical pathology selected abstracts
February 2019—Impact of commercial laboratory testing on a care delivery system: Care delivery systems have become increasingly fragmented and complex, which impacts patient care. The amount of health care data generated has also created a problem by reducing the time devoted to direct patient care.
Read More »Anatomic pathology selected abstracts
February 2019—Diagnostic algorithmic proposal based on IHC evaluation of invasive endocervical adenocarcinomas: The International Endocervical Adenocarcinoma Criteria and Classification was developed to separate endocervical adenocarcinomas into two main categories based on morphology: human papilloma virus-associated (HPVA) and nonhuman papilloma virus-associated adenocarcinomas.
Read More »Molecular pathology selected abstracts
February 2019—Evaluation of mutational processes and somatic driver mutations in cancer exomes: As next-generation sequencing-based tumor profiling gains popularity, multiple informative variables other than single-gene alterations will continue to be added to clinical reports.
Read More »Newsbytes
February 2019—Digital pathology RFPs: from the questions to selections: To those who request the information and those who supply the information, requests for proposal, better known as RFPs, can be groanworthy. Yet laboratories planning to purchase a digital pathology system for clinical use should seriously consider going through the painstaking process, even if their institutions don’t require it, says Liron Pantanowitz, MD, vice chair of pathology informatics at the University of Pittsburgh Medical Center.
Read More »Taking measure of cholangiocarcinoma
January 2019—Ten years ago, says Manhal Izzy, MD, the approach might have seemed quixotic: performing liver transplants in patients with early intrahepatic cholangiocarcinoma. Even today, it’s hardly standard of care. Nevertheless, it has moved well beyond the impossible dream category. Medicine advances, and practices change. There’s nothing unusual about that. But Dr. Izzy, assistant professor of medicine, Vanderbilt University School of Medicine, and transplant hepatologist, Vanderbilt University Medical Center, goes out of his way to credit the wisdom and work of those in hepatology and oncology who have been pushing forward curative approaches for cholangiocarcinoma.
Read More »Navigating the quandaries of coagulation testing
January 2019—Naming the things about coagulation testing that most perplex clinicians isn’t easy for Michael Laposata, MD, PhD. But there’s a good reason for that: He finds confusion to be pervasive.
Read More »At-home testing for heart failure, transplant patients: Can it work?
January 2019—Medicare hospital readmission rates are down under the Centers for Medicare and Medicaid Services readmissions reduction program, though hospitals are still paying millions in penalties, in part because new conditions are included in the calculations.
Read More »Drug-susceptibility testing for TB: poised to take a turn?
January 2019—In a large international study, whole genome sequencing with next-generation sequencing technology has proved its ability to accurately assess susceptibility of Mycobacterium tuberculosis isolates to four first-line drugs.
Read More »Parasites in tissue: how to identify the structures
January 2019—Pathologists who aren’t microbiologists can provide a diagnosis of parasitic disease if they take into account parasite life cycles and tissue tropisms. Julie A. Ribes, MD, PhD, made that key point in cases she presented in her CAP18 session, “Update on Invasive Parasitic Infections for Surgical Pathologists.” Dr. Ribes added learning material to most of the cases, she said, but the cases come from parasites she has seen and known in her own professional life.
Read More »AMP case report: Identification by NGS of a diagnostic and theranostic mutation in a high-grade sarcoma of the humerus
January 2019—A 75-year-old woman with history of melanoma localized to the right forearm and status post excision six years prior presented with a two-month history of continuous left shoulder pain. She was managed initially with physical therapy and hydrocodone with no effect. Initial workup at an outside institution included an x-ray of the left shoulder that showed a destructive lytic lesion involving the proximal aspect of the left humerus associated with a pathologic fracture.
Read More »Cytopathology in focus: How can a lab ensure individual competence?
January 2019—It is happening again: CAP members and cytotechnologists are asking about regulatory requirements for re-integrating into cytopathology after a period of practice latency. That is good news because it indicates that they are interested in practicing at a time when the cytopathology community can use skilled professionals. The past decade has seen a shrinking volume of Pap tests and a concomitant decline in the number of practicing cytologists, which has created new job opportunities for those with cytopathology skills.
Read More »Cytopathology in focus: Serous fluid cytology and the international system
January 2019—Just when you thought you were done implementing a new terminology for cytology, another one pops up. Is it possible there are sites that have not yet been standardized? Unbelievably, the most common nongynecologic cytology specimen, body fluids, remains a Wild West for terminology.
Read More »Cytopathology in focus: Next-generation cytotechnology—new cytotechnologist roles
January 2019—The evolution of minimally invasive techniques and new diagnostic modalities have placed new demands on medical laboratories. Cytotechnologists find themselves uniquely poised to take on these new responsibilities, using their morphologic and analytical skills. In this article, I summarize potential roles for cytotechnologists that go beyond screening cytology slides to add value and improve quality in the clinical laboratory.
Read More »Cytopathology in focus: Three special reports capture a field in transition
January 2019—In the September/October 2018 issue of the Journal of the American Society of Cytopathology are three special reports from the American Society of Cytopathology/American Society for Clinical Pathology workgroup on current practices and future perspectives for the field of cytotechnology.
Read More »Cytopathology in focus: ABPath CertLink pilot open to all
January 2019—The American Board of Pathology subspecialty certification examination in cytopathology is given each fall at the ABPath office in Tampa, Fla. Cytopathology has the largest number of examinees of the 11 pathology subspecialty examinations.
Read More »Put It on the Board
FDA clears 2 of 3
ePlex blood culture ID panels:
January 2019—GenMark Diagnostics announced in December that it received FDA market clearance for its ePlex Blood Culture Identification Gram-Positive (BCID-GP) and Fungal Pathogen (BCID-FP) panels. GenMark’s third panel, ePlex Blood Culture Identification Gram-Negative (BCID-GN), was submitted to the FDA in September 2018 and is still under review. The fungal pathogens panel has broad coverage and includes many resistant and emerging strains, among them Candida auris, GenMark said in its statement. The company says that by coupling BCID panels with the ePlex Templated Comments software module, hospitals can enable immediate intervention linked to a diagnostic result and improve the effectiveness of antimicrobial stewardship initiatives.
12 assays for Atellica Solution:
Siemens Healthineers achieved 12 pre-market approvals from the FDA for its Atellica Solution infectious disease and oncology testing menu.
Q&A column
Q. Which gynecological slides can cytotechnologists report? Read answer. Q. Is it recommended that a hospital streamline its reference ranges for point-of-care and main laboratory tests? Read answer.
Read More »Newsbytes
January 2019—Virtual tumor board platforms: a game changer for cancer case review: If Suneal Jannapurredy, MD, had been able to read the patient’s outside radiology report prior to breast tumor board, he would have re-examined the gross specimen to determine whether, as the other providers were now telling him, there was a second area of focus he hadn’t included in his presentation.
Read More »From the President’s Desk: Practice engagement resources for all
January 2019—Excellence in the laboratory can have a powerful impact on the culture of our institutions because we come into contact with so many and so much. Mostly, we just need to do complex things extremely well and make it look easy. In other words, practice pathology. The more than 50 pathologists and numerous laboratory professionals in my group practice, Delta Pathology, serve nearly 100 institutions across Louisiana and Mississippi.
Read More »Clinical pathology selected abstracts
January 2019—RH genotype matching for transfusion support in sickle cell disease: In patients with sickle cell disease, Rh alloimmunization remains a challenge, despite the transfusion of serologic Rh C, E, and K antigen-matched red cells.
Read More »Anatomic pathology selected abstracts
January 2019—Distinguishing patients with double somatic mismatch-repair mutations from those with Lynch syndrome: Lynch syndrome is the most common form of hereditary colon cancer. Germline mutations in the mismatch-repair (MMR) genes MLH1, MSH2 (EPCAM), MSH6, and PMS2, followed by a second hit to the remaining allele, lead to cancer development.
Read More »Molecular pathology selected abstracts
Editors: Donna E. Hansel, MD, PhD, chief, Division of Anatomic Pathology, and professor, Department of Pathology, University of California, San Diego; James Solomon, MD, PhD, resident, Department of Pathology, UCSD; Richard Wong, MD, PhD, molecular pathology fellow, Department of Pathology, UCSD; and Sounak Gupta, MBBS, PhD, molecular pathology fellow, Memorial Sloan Kettering Cancer Center, New York. Development of brain circuits ...
Read More »Daniel J. Hanson, MD, 1928–2018
January 2019—Daniel J. Hanson, MD, a member of the CAP Board of Governors from 1993 to 1999, died on Nov. 2, 2018. Dr. Hanson was president and medical director of Pathology Laboratories in Toledo, Ohio, until he retired in 2005. He was also clinical professor of pathology emeritus at the University of Toledo College of Medicine.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management