 CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the Laboratory of Genomic Medicine, Department of Pathology, University of Illinois at Chicago. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the Laboratory of Genomic Medicine, Department of Pathology, University of Illinois at Chicago. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Erica Vormittag-Nocito, MD
Tibor Valyi-Nagy, MD, PhD
Gayatry Mohapatra, PhD
September 2019—Primary bladder adenocarcinoma is a rare vesicle malignancy accounting for up to two percent of malignant neoplasms of the bladder.1 They occur in males more than females and are classically seen in the fifth or sixth decade of life.2 Histologically they are of enteric, mucinous, or mixed types. Morphologically, the enteric type appears identical to a colonic adenocarcinoma and the mucinous type appears as neoplastic cells floating in pools of extravasated mucin. The mixed type is a mixture of the morphologies of the enteric and mucinous types. Immunohistochemically, adenocarcinomas of the urinary bladder classically express CK20 and CDX2. This tumor type has a poor prognosis with a low five-year overall survival of approximately 50 percent.1 Herein we describe a young adult male with metastatic primary bladder adenocarcinoma with interesting molecular alterations.
Case. A 25-year-old male presented to the emergency room for progressive bilateral lower extremity weakness and right leg paresthesia. He had a past medical history for bladder cancer of unknown type treated by partial cystectomy at age 20 at an outside hospital followed by chemotherapy that was stopped due to intolerance. Four years after initial treatment he was found to have bone metastases in multiple areas of the axial and appendicular skeleton. Chemotherapy followed by immunotherapy was initiated. Radiation therapy was also used for control of the metastatic disease. Most recently he had additional radiation therapy for lung metastasis one month before presenting to our institution. On current presentation, an MRI of the spine revealed an enhancing soft tissue mass at L1 with compression of the distal spinal cord. The patient underwent laminectomy with tumor resection.
The histologic sections of the spinal resection showed islands of neoplastic gland-forming cells within pools of extravasated mucin within the bone marrow cavity (Figs. 1A and 1B). The neoplastic cells exhibit pleomorphism and hyperchromasia of the nuclei. Goblet cells were identified within the neoplastic clusters. Immunohistochemically, these neoplastic cells showed patchy positivity for CK20 (Fig. 1C) and CDX2 (Fig. 1D) and negativity for CK7 and TTF-1. A beta-catenin IHC, to rule out colonic origin, was not performed as the patient had an extensive clinical history of metastatic urinary bladder carcinoma. Additional masses suggestive of a second primary lesion were not identified on MRI. A diagnosis of metastatic mucinous adenocarcinoma was made, and the case was sent for further molecular studies.
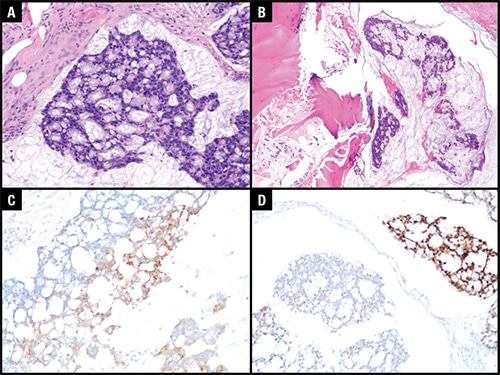
Fig. 1. A. H&E-stained image at 200× magnification of neoplastic cells floating in extravasated mucin pool. B. H&E-stained image at 100× magnification showing involvement of tumor in the vertebral bone marrow cavity. C. Immunohistochemical stain of CK20 at 200× magnification showing patchy positivity. D. Immunohistochemical stain of CDX2 at 200× magnification showing patchy positivity.
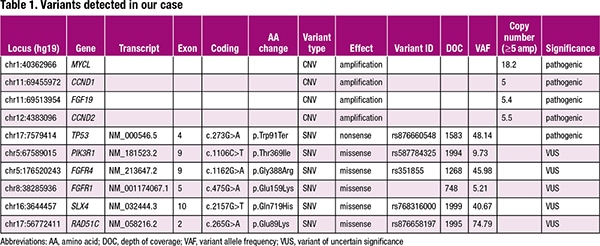
A pathologist (EVN) reviewed an H&E section to estimate tumor cellularity. The area containing solid viable tumor was circled for macrodissection. The percent tumor cells in the circled region was estimated to be 100 percent. DNA and RNA were extracted from unstained formalin-fixed, paraffin-embedded sections and analyzed by next-generation sequencing using the Oncomine Comprehensive Assay v3 (OCAv3) (Thermo Fisher Scientific). Library preparation, emulsion PCR, and chip loading were performed using the Ion Chef System. Sequencing was performed on an Ion 540 chip using the Ion S5 XL sequencer (Thermo Fisher Scientific). Sequencing reads were mapped to the University of California Santa Cruz human genome build GRCh37/hg19 using Torrent Suite software (version 5.10; Thermo Fisher Scientific). Data analysis and variant calling were performed using a procedure reported previously.3 The overall depth of coverage was 2439×. NGS results are presented in Table 1. Of note, a CCND1 amplification, MYCL amplification, and TP53 p.W91* pathogenic variant were detected, among others (Table 1).
Discussion. Primary mucinous bladder adenocarcinoma is a rare neoplasm with an aggressive clinical course and overall poor prognosis.4 The differential diagnosis in this case would include metastatic colorectal cancer, primary bladder mucinous type adenocarcinoma, and urachal carcinoma. Morphologically and immunohistochemically, it can be difficult to distinguish these entities from each other. Morphologically, all can show a mucinous variant, and immunohistochemically CK7 and CK20 staining in all these entities can be variable.5 In addition, CDX2, which was initially thought to be specific for colon adenocarcinomas, can also be positive in primary bladder carcinomas.5 β-catenin has been the only marker in the literature that can consistently differentiate colorectal adenocarcinoma from primary bladder urothelial carcinomas.5 As the β-catenin pathway is altered in colonic adenocarcinomas, nuclear expression of β-catenin is seen in colonic primary adenocarcinoma.5 As this protein is not disrupted in primary bladder adenocarcinomas of urachal and non-urachal origin, immunohistochemically membranous expression is seen.5 Unfortunately, urachal carcinomas may stain similarly to both primary bladder non-urachal adenocarcinomas and colorectal adenocarcinomas, making the differential diagnosis difficult in practice.6
It has been suggested that urachal and non-urachal bladder adenocarcinomas may arise through different molecular pathways. In a small study by Kardos, et al., it was shown by RNA expression analysis that urachal carcinomas have alterations similar to colorectal adenocarcinomas and glioblastomas.7 These tumors show high frequency of mutations in APC, MTOR, NF1, MLL3, and ARID4B, all of which are not mutated at high frequency in urothelial and non-urachal adenocarcinomas.8 Larger cohort studies need to be performed to confirm the differences in these diagnostically difficult rare neoplasms.
Although prognostic studies on primary bladder adenocarcinomas have not been published in the literature, there are a few published studies regarding molecular prognostic indicators in urothelial carcinoma on the alterations seen in our case of mucinous adenocarcinoma. Of note, CCND1 amplification has been described as one of the more common amplifications found in the urothelial carcinoma cohort from the Cancer Genome Atlas Research Network.8 CCND1 is a key regulator of cell cycle. It encodes the protein CyclinD1, which ultimately targets the protein Rb for phosphorylation and inactivation.9 It has been shown that amplification of this gene portends a poor prognosis in many types of carcinoma.10 Although CCND1 amplification portends a poor prognosis, these urothelial tumors show better response to chemotherapy.10 In addition, the interaction of CyclinD1 and the cyclin-dependent kinases Cdk4 and Cdk6 are potential targets for new chemotherapeutic agents.
Urothelial carcinomas have also shown high rates of FGFR3 and TP53 mutations.8,9 These two alterations in urothelial carcinoma are thought to be related to low-grade papillary carcinoma development and high-grade urothelial carcinoma, respectively.9 These mutations are not mutually exclusive and both can be seen in cases where a low-grade lesion progresses to a higher-grade muscle-invasive carcinoma. Unfortunately, although these mutations may be related to carcinogenesis, no targeted therapy or prognostic implications can be made about these mutations at this time.
In our case of metastatic mucinous carcinoma with a history of bladder primary tumor, molecular results correlate to a primary bladder adenocarcinoma, non-urachal mucinous type. As these are rare tumors, a larger cohort needs to be studied to better understand the underlying biology of these tumors and to identify novel prognostic/therapeutic markers.
- Grignon DJ, Cheville J, Ro JY, Tamboli P. Glandular neoplasms. In: Moch H, Humphrey PA, Ulbright TM, Rueter VE, eds. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Lyon, France: IARC Press; 2016:111–112.
- Vasudevan G, Bishnu A, Singh BMK, Nayak DM, Jain P. Bladder adenocarcinoma: a persisting diagnostic dilemma. J Clin Diagn Res. 2017;11(3):ER01–ER04.
- Acosta AM, Al Racheed MRH, Pins MR, et al. The role of next-generation sequencing in the differential diagnosis of composite neoplasms. Hum Pathol. 2018;81:78–88.
- Baffigo G, Delicato G, Bianchi D, et al. Mucinous adenocarcinoma of the urinary bladder. Am J Case Rep. 2012;13:99–101.
- Roy S, Parwani AV. Adenocarcinoma of the urinary bladder. Arch Pathol Lab Med. 2011;135(12):1601–1605.
- Taylor AS, Mehra R, Udager AM. Glandular tumors of the urachus and urinary bladder: a practical overview of a broad differential diagnosis. Arch Pathol Lab Med. 2018;142(10):1164–1176.
- Kardos J, Wobker SE, Woods ME, et al. Comprehensive molecular characterization of urachal adenocarcinoma reveals commonalities with colorectal cancer, including a hypermutable phenotype. JCO Precis Oncol. Epub July 6, 2017. doi:10.1200/PO.17.00027.
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322.
- Ross JS, Wang K, Al-Rohil RN, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2014;27(2):271–280.
- Seiler R, Thalmann GN, Rotzer D, Perren A, Fleischmann A. CCND1/CyclinD1 status in metastasizing bladder cancer: a prognosticator and predictor of chemotherapeutic response. Mod Pathol. 2014;27(1):87–95.
Dr. Vormittag-Nocito is a resident, Dr. Valyi-Nagy is a professor of pathology and director of neuropathology, and Dr. Mohapatra is director of genomic medicine—all in the Laboratory of Genomic Medicine, Department of Pathology, University of Illinois at Chicago.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
