CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, Mumbai, India. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Sewanti Limaye, MBBS, MD, MS; Madhavi Pusalkar, PhD; Foram Kothari, MBBS; Meenal Hastak, MBBS, MD; Varsha Vadera, MBBS, MD; Rajesh Mistry, MBBS, MS; Jaya Vyas, PhD
 May 2019—Non-small cell lung cancer patients with epidermal growth factor receptor activating mutations have excellent response to oral therapy with EGFR tyrosine kinase inhibitors. However, development of resistance to first- and second-generation TKIs is a well-recognized phenomenon with acquired p.T790M mutation and accounts for most TKI drug resistance. Resistance to EGFR TKI therapy has been described in tumors with coexistent primary p.T790M mutation and an EGFR activating mutation in a small number of patients. These patients with coexistent EGFR activating mutation and primary p.T790M mutation have been reported to have less than adequate clinical benefit from first- and second-generation EGFR TKI therapy.1-3
May 2019—Non-small cell lung cancer patients with epidermal growth factor receptor activating mutations have excellent response to oral therapy with EGFR tyrosine kinase inhibitors. However, development of resistance to first- and second-generation TKIs is a well-recognized phenomenon with acquired p.T790M mutation and accounts for most TKI drug resistance. Resistance to EGFR TKI therapy has been described in tumors with coexistent primary p.T790M mutation and an EGFR activating mutation in a small number of patients. These patients with coexistent EGFR activating mutation and primary p.T790M mutation have been reported to have less than adequate clinical benefit from first- and second-generation EGFR TKI therapy.1-3
We report here a case of advanced adenocarcinoma of the lung with primary p.T790M mutation and an EGFR exon 19 deletion that showed response to the third-generation EGFR TKI osimertinib.
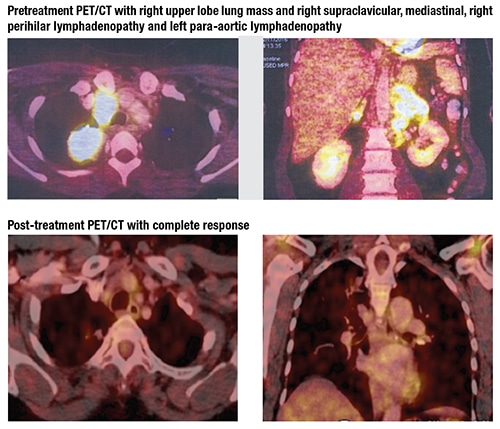
Case. A 54-year-old Indian woman, otherwise in good health and with no tobacco exposure, presented to our hospital in November 2016 with a right neck lump and persistent cough of one-month duration. Fine-needle aspiration cytology was suspicious for squamous cell carcinoma. The patient underwent staging using PET/CT scan, which revealed a right upper lobe lung mass and right supraclavicular, mediastinal, right perihilar, and left para-aortic lymphadenopathy. A core biopsy was performed from the right supraclavicular lymph node and revealed poorly differentiated adenocarcinoma of lung primary.
At the time of the patient’s evaluation in 2016, only EGFR mutation analysis using Sanger sequencing and FISH using ALK break-apart strategy were being performed as part of routine molecular testing of lung adenocarcinoma cases. The patient’s tumor specimen was negative for ALK rearrangement by FISH.
Sanger sequencing, the gold-standard technique, has the advantage of detecting all the mutations—common ones known to be TKI sensitive and resistant and uncommon ones whose clinical sensitivity to TKIs is not clear. The tumor content of the biopsy tissue in this patient was 30 percent, which is the lower cutoff limit for Sanger sequencing at our center.
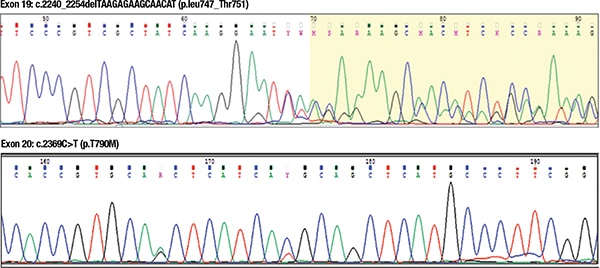
 DNA was extracted from the tissue obtained from the core biopsy sample using a QIAamp FFPE kit (Qiagen). Quantified DNA was amplified by polymerase chain reaction using primers specific for exons 18, 19, 20, and 21 of EGFR. Obtained PCR products were sequenced by bidirectional DNA sequencing on an ABI capillary sequencer.4 Result analysis revealed two mutations in the EGFR gene, one in exon 19 (p.L747_T751del) and the other in exon 20 (p.T790M).
DNA was extracted from the tissue obtained from the core biopsy sample using a QIAamp FFPE kit (Qiagen). Quantified DNA was amplified by polymerase chain reaction using primers specific for exons 18, 19, 20, and 21 of EGFR. Obtained PCR products were sequenced by bidirectional DNA sequencing on an ABI capillary sequencer.4 Result analysis revealed two mutations in the EGFR gene, one in exon 19 (p.L747_T751del) and the other in exon 20 (p.T790M).
Discussion. Detection of a compound mutation in the EGFR gene is a rare occurrence. Nearly one percent to seven percent of patients have been reported to have compound mutations.5,6 Detection of one sensitive and one resistant mutation is an interesting and complicated situation. Although exon 21 and exon 20 mutations have been reported as one of the common compound mutations, the combination of exon 19 and exon 20 is rare and has been reported in about 0.5 percent of cases in one study.7 Clinical response of these rare cases to TKI inhibitors is not well studied.
In light of previous data suggesting a lack of appropriate clinical benefit with EGFR TKI therapy in primary p.T790M mutation1-3 and the large disease burden seen in this symptomatic patient, a decision was made to treat her with standard chemotherapy doublets and not with first- or second-generation EGFR TKIs. The patient received intravenous pemetrexed 500 mg/m2 and carboplatin 550 mg every three weeks for a total of six cycles, followed by maintenance pemetrexed 500 mg/m2 for six additional cycles. A restaging PET/CT after three cycles of chemotherapy revealed a partial response. The patient experienced significant and progressive adverse effects with pemetrexed in the form of a grade two skin rash, grade two fluid retention, and grade three right submandibular gland inflammation despite steroid premedication and anti-allergic administration.
 The third-generation EGFR TKI osimertinib is known to work both on EGFR exon 19 deletion and secondary p.T790M mutation8 and was launched in India in November 2017. Due to toxicity from maintenance pemetrexed and persistent disease, our patient with EGFR exon 19 deletion and primary p.T790M mutation was started right away on osimertinib 80-mg tablets once daily. She has completed six months of therapy without any significant adverse effects and with complete response to therapy on the most recent PET/CT. Even the inflammation in the right submandibular gland has resolved completely.
The third-generation EGFR TKI osimertinib is known to work both on EGFR exon 19 deletion and secondary p.T790M mutation8 and was launched in India in November 2017. Due to toxicity from maintenance pemetrexed and persistent disease, our patient with EGFR exon 19 deletion and primary p.T790M mutation was started right away on osimertinib 80-mg tablets once daily. She has completed six months of therapy without any significant adverse effects and with complete response to therapy on the most recent PET/CT. Even the inflammation in the right submandibular gland has resolved completely.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
