
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the National University Health System, Singapore. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Rachel Su Jen Wong, MBBS; Anand Jeyasekharan, MBBS;
Xinyi Su, MBB Chir, PhD, MMed; Kok Siong Poon, MSc;
Soo Yong Tan, MBBS, DMJ, DPhil; Gopal Lingam, MBBS, MS;
Benedict Yan, MBBS
October 2019—Primary intraocular lymphoma (PIOL) is a rare but aggressive B-cell malignancy usually considered as a subtype of primary central nervous system lymphoma.1-4 The most common form of PIOL is primary vitreoretinal lymphoma. PIOL is also known as the masquerade syndrome4 because it frequently mimics other ocular conditions such as chronic uveitis, which may be steroid-resistant.4,5 Its diagnosis is challenging and requires a high degree of suspicion. Here, we present a case of PIOL, the diagnosis of which was clinched based on the identification of a mutation in the myeloid differentiation factor 88 (MYD88) gene.
Case. A 52-year-old Malay female presented with a two-year history of bilateral blurring of vision, worse in the left eye. There were no other systemic or B symptoms, no abnormal lumps/bumps felt, and no personal or family history of cancer. Apart from a history of ureteric colic, which was conservatively managed three years prior to her presentation, she had no other significant medical history, nor was she on any long-term medications, immunosuppressants in particular.
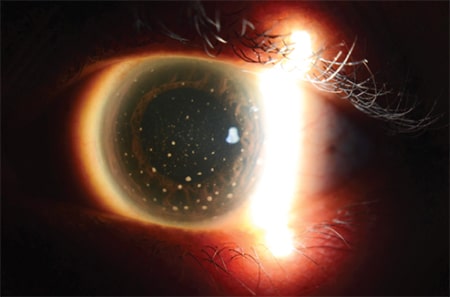
Fig. 1. Keratic precipitates seen on the cornea.
Slit-lamp examination of the eye revealed the presence of keratic precipitates on the corneal endothelium (Fig. 1), active anterior chamber activity, and dense vitritis consistent with panuveitis. Paracentesis of the anterior chamber was performed and the aqueous fluid sent for infective screening. This included pathogen-directed polymerase chain reaction analysis for tuberculosis mycobacterium, cytomegalovirus, herpes simplex virus, varicella-zoster virus, and Toxoplasma gondii, all of which were negative. A full blood count did not show any leukocytosis, C-reactive protein was low (1.5 mg/L), and erythrocyte sedimentation rate was only slightly raised for her age (34 mm/hr). Screening from serum for syphilis and TB were also negative.
She was treated with a course of topical and oral steroids for uveitis with minimal improvement in symptoms. Further workup for uveitis, including testing for antinuclear antibodies, lupus anticoagulant antibodies, and antineutrophil cytoplasmic antibodies, did not yield a diagnosis. She was counseled for a diagnostic left eye vitrectomy, which she successfully underwent. Intraoperative findings showed left eye chronic vitritis, with dense sheets of vitreous and cellular deposits on the inferior retinal surface. Five milliliters of vitreous fluid were sampled, and cytology revealed a small number of small and large lymphocytes, some of which showed marked irregularity of nuclear outlines and contained coarse chromatin. Immunocytochemical testing (Fig. 2) showed many CD20-positive B-lymphocytes, and multiple small T-lymphocytes, which stained CD3 positive. Ki-67 was positive in most of the larger cells. The diagnosis of large B-cell lymphoma was suspected; however, it was not histologically confirmed in view of the limited yield.
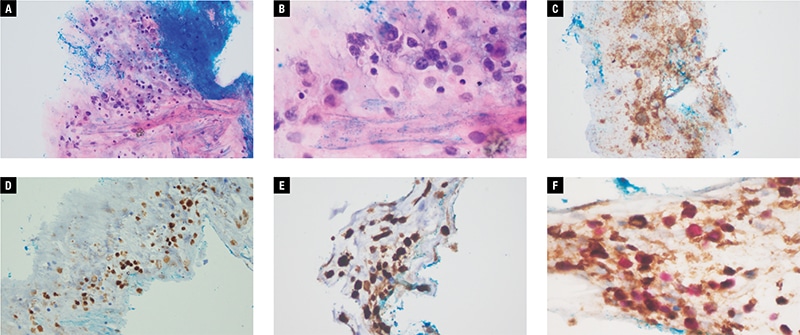
Fig. 2 (A–F). Photomicrographs of vitreous fluid sample with: A. Hematoxylin-eosin stain at 200 × magnification; B. Hematoxylin-eosin stain at 400 × magnification; C. CD20 stain; D. PAX5 stain; E. Ki-67 stain; CD79A (brown) Ki-67 (red) stain.
Subsequent staging workup was performed, with plans to identify other areas of lymphomatous disease from which additional biopsies could be obtained to help confirm the diagnosis. While a whole body PET CT scan showed symmetrical FDG-avidity in the bilateral posterior nasopharynx and palatine tonsils, a biopsy of the post-nasal space was negative for malignancy. A contrasted magnetic resonance imaging of the brain did not yield any abnormal enhancing lesion; lumbar puncture showed an acellular smear, and a bone marrow biopsy revealed a normo-cellular marrow negative for any lymphomatous involvement. The patient was understandably not keen for a repeat vitrectomy in the other eye to attempt to confirm the diagnosis, especially since there remained a possibility of inadequate yield for histological confirmation of disease again. We therefore pursued a molecular analysis of the vitreous fluid obtained during the vitrectomy. Genomic DNA was extracted from the patient’s vitreous fluid sample and assayed for the MYD88 p.Leu265Pro mutation using pyrosequencing, which was positive (Figs. 3 and 4), to allow final confirmation of diagnosis.
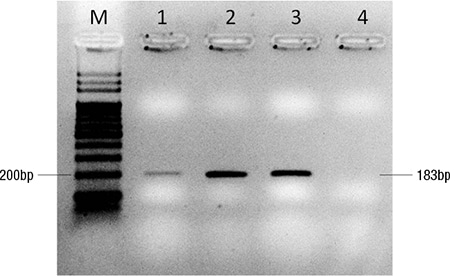
Fig. 3. Genomic DNA was extracted by Gentra Puregene Blood Kit (Qiagen, GmbH, Germany) and subject to PCR amplification. Electrophoresis was performed on the PCR products using two percent agarose gel. M: 100 base pair DNA ladder; 1: Patient sample; 2: 100 percent mutant DNA control; 3: 100 percent wild-type DNA control; 4: Non-template control.
Discussion. This case highlights the difficulty and challenge in diagnosing PIOL given the vague symptoms, similar presentation to chronic uveitis,2 and the small amount of tissue sample that is usually obtained from biopsy.6 PIOL presents with nonspecific symptoms such as blurring of vision, decreased visual acuity, and floaters, and it tends to occur bilaterally in 60 to 90 percent of patients, though often asymmetrically.4 The many common masqueraders include sarcoidosis, tuberculosis, viral retinitis, and syphilis.3 It is usually diagnosed by cytology, immunohistochemistry, and molecular examination of vitreous body samples, including that of gene rearrangements in the immunoglobulin heavy chain (IGH) frequently seen in B-cell lymphoma.1,2,6 The ability to clinch a diagnosis with the use of MYD88 mutational analysis helps to reduce the need for repeat biopsies and allow appropriate treatment to be instituted early.
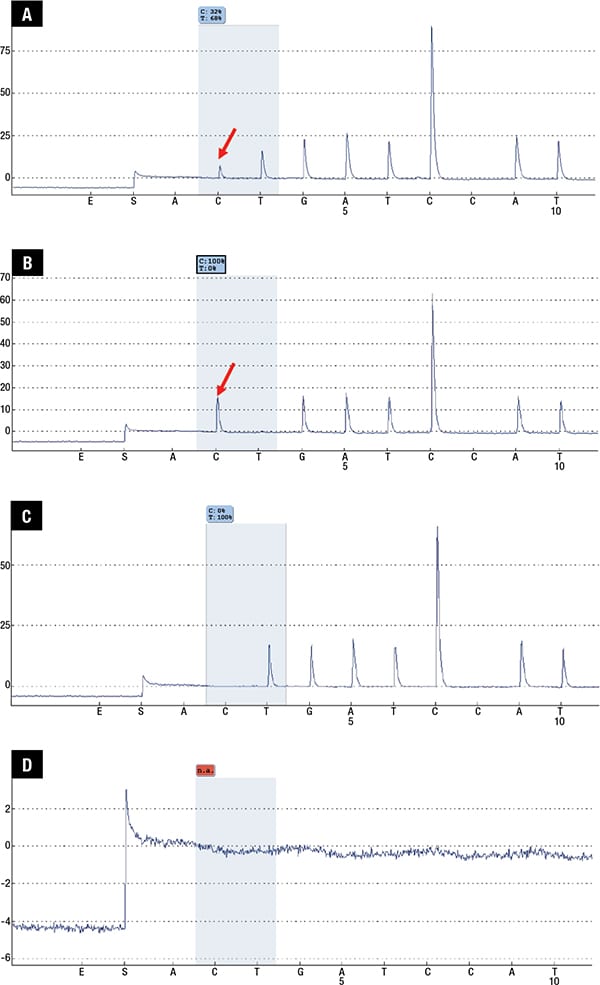
Fig. 4 (A–D). PCR products purified by PyroMark Q96 Vacuum Workstation (Qiagen, GmbH, Germany) were subject to pyrosequencing on PyroMark Q96 ID instrument (Qiagen). Analysis was performed using PyroMark Q96 ID software (Qiagen). Pyrograms with quantitative mutant allele burden were displayed. A. Patient sample; B. 100 percent mutant DNA control; C. 100 percent wild-type DNA control; D. Non-template control.
This is especially crucial as PIOL is associated with poor prognosis and high rates of relapse.3,4 It is considered a subtype of primary central nervous system lymphoma, though only 15 to 25 percent of patients with PCNSL will have ocular involvement.2,3 Mutations in the MYD88 gene (usually the canonical L265P mutation) have been found in 35 to 79 percent of PCNSL, and recently identified in approximately 70 percent of primary vitreoretinal lymphoma with or without concomitant cerebral involvement.1,4 Indeed, the presence of the L265P mutation in the MYD88 gene has been studied and shown to be an effective tool in the diagnosis of PIOL, especially in cases when there is minimal/poor material,6 as highlighted in this case, whereby only ocular involvement was observed without additional systemic lesions for biopsy. Only 10 to 20 ng of genomic DNA is required for pyrosequencing,7 whereas the detection of gene rearrangement in IGH via microdissection and subsequent PCR requires at least 15 atypical lymphoid cells.8 Our patient has since been started on systemic chemotherapy with methotrexate, rituximab, procarbine, and vincristine, with improvement in symptoms.
- Fend F, Ferreri AJ, Coupland SE. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol. 2016;173(5):680–692.
- Zhou M, Xu G. Recent progress in the diagnosis and treatment of primary vitreoretinal lymphoma. Taiwan J Ophthalmol. 2016;6(4):170–176.
- Kapelko-Slowik K, Urbaniak-Kujda D, Turno-Krecicka A, et al. Primary intraocular diffuse large B-cell lymphoma: diagnostic difficulties in deep retinal infiltrations with vitritis. Indian J Hematol Blood Transfus. 2016;32(suppl 1):143–147.
- Araujo I, Coupland SE. Primary vitreoretinal lymphoma—a review. Asia Pac J Ophthalmol (Phila). 2017;6(3):283–289.
- Sagoo MS, Mehta H, Swampillai AJ, et al. Primary intraocular lymphoma. Surv Ophthalmol. 2014;59(5):503–516.
- Bonzheim I, Giese S, Deuter C, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126(1):76–79.
- Cummings PJ, Ahmed R, Durocher JA, Jessen A, Vardi T, Obom KM. Pyrosequencing for microbial identification and characterization. J Vis Exp. 2013;(78):e50405. doi:10.3791/50405.
- Wang Y, Shen D, Wang VM, Sen HN, Chan CC. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int J Mol Sci. 2011;12(9):5684–5697.
Dr. Wong is in the Department of Medicine, Dr. Jeyasekharan is in the Department of Hematology-Oncology, Dr. Su and Dr. Lingam are in the Department of Ophthalmology, Kok Siong Poon and Dr. Yan are in the Molecular Diagnosis Centre, and Dr. Tan is in the Department of Pathology—all in the National University Health System, Singapore.
Test yourself
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
