IGHV mutation analysis should be top of mind when acquiring prognostic and potentially therapeutic information in chronic lymphocytic leukemia, said Curtis A. Hanson, MD, in a CAP TODAY webinar made possible by a special educational grant from Diaceutics. Dr. Hanson, professor of laboratory medicine and pathology, Mayo Clinic College of Medicine, explained the structure and function of IGHV, the mutation assay, and the clinical value of mutation status. Here is an edited transcript of what he said.
May 2019—Chronic lymphocytic leukemia is a neoplasm of small mature B-cells and the most common leukemia diagnosed in adults. Median age of diagnosis is 70 years, but there is a surprisingly large percentage of patients, about 10 percent, who are younger than 55, and it’s not uncommon now to occasionally see CLL patients, about two percent, in their 40s.

Dr. Hanson
Although the overall survival rate is good, there is a lot of variability in patients with CLL, ranging from indolent and slowly progressive to others who have a more progressive course that requires aggressive treatment. That has been the holy grail in CLL: trying to identify those who will develop aggressive disease and treating them with appropriate therapy.
The first important step was developing a large number of prognostic markers that were useful in establishing risk categories. The next step, which has taken place over the past few years, is that new therapies for progressive disease in CLL are expanding, with improvements seen in overall survival, even for patients whom we previously would have put into a high-risk group.
Pathologists see CLL patients at several points in the course of their disease. The first step in the CLL evaluation process is to suspect it or recognize it. That might be because of a peripheral lymphocytosis, adenopathy, organomegaly, or, not uncommonly, an incidental pathology finding. The second step is to make the diagnosis, which is dependent not only on flow cytometric immunophenotyping but also, from a surgical pathology and hematopathology perspective, on tissue biopsies.
Step three is to subclassify it, making sure we obtain the right prognostic information, which will drive therapy, with the primary findings now from a pathology and laboratory perspective being the mutation status of the immunoglobulin heavy-chain variable region gene (IGHV) and CLL FISH studies.
During patient follow-up (step four), we need to be able to detect residual disease, and minimal residual disease studies by flow cytometry are the current standard. Finally, when we move into disease progression, the most important assays now center around TP53 mutation, by FISH and sequencing.
The historic staging systems in CLL have been the Rai (modified) and Binet systems. And the key immunophenotype is CD5 co-expression with a variety of pan-B cell antigens—CD19, CD20, and CD23.
CD5 is also a characteristic finding in mantle cell lymphoma, which we can confirm with genetic studies or immunostains for CCND1. Importantly, 10 to 20 percent of marginal zone and lymphoplasmacytic lymphomas may also express CD5 and, if not considered, can easily be confused and misdiagnosed as CLL when those two disorders involve the peripheral blood and bone marrow.
When we do encounter immunophenotypes that are not classic for CLL, it’s critical that we recommend to our clinicians that they consider a lymph node or other tissue biopsy so we can be sure we will have the right diagnosis, as opposed to arbitrarily pushing everything into a CLL diagnosis.
The classification scheme for patients who have a CLL-like immunophenotype is simple. Two criteria are needed: knowing what the absolute clonal B-cell count is, and the presence or absence of adenopathy. For those who have greater than 5,000 clonal B-cells, it’s simple: Regardless of adenopathy status, it’s CLL. For those who have less than 5,000 B-cells and no adenopathy, it’s monoclonal B-cell lymphocytosis. With adenopathy, it’s small lymphocytic lymphoma.
The development of prognostic markers has been one of the two biggest improvements in CLL. Unmutated IGHV gene is a molecular marker associated with poorer prognosis and shorter survival (mean OS = 95 months). Forty to 50 percent of patients will have the unmutated IGHV gene. The remainder are mutated; the prognosis is good and the mean overall survival is 293 months.It’s important also to look for TP53 point mutations. This is related to, but separate from, looking for P53 deletions by FISH. The incidence at diagnosis is five to 10 percent.
CLL FISH studies cannot be used to diagnose CLL because the anomalies—13q, trisomy 12, 11q, and 17p (p53)—can be seen in other lymphoproliferative disorders.
To discuss the clinical value of IGHV mutation status, I’m going to begin with an early history. It was known that the Rai and Binet staging systems did not recognize the biological diversity of CLL or predict response to modern therapy. Today the most important prognostic system in CLL is the CLL International Prognostic Index, or CLL-IPI. But before the CLL-IPI, a simple prognostic system had been proposed based on only two markers: FISH testing and IGHV mutation status, with patients separated into three risk groups. So it was recognized early on that the IGHV gene was going to be an important prognostic marker.
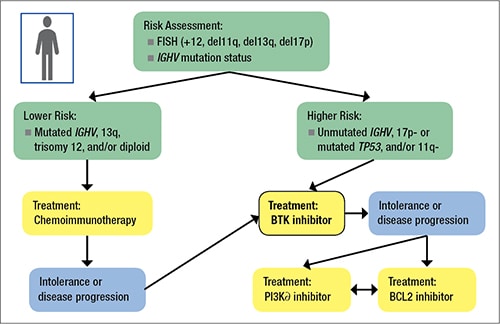
Fig. 1. Typical application of “risk” in a CLL patient
The flow-based prognostic markers—CD38, ZAP70, and CD49d—were easy to do and provided a lot of information but have not stood up over time as independent markers and are not required, in my view, in the evaluation of CLL patients today. There is also a variety of serum-based prognostic markers: soluble CD23, thymidine kinase, and β2-microglobulin. In several studies performed over time, β2-microglobulin has retained its independent prognostic value.
Let’s walk through how to apply that typical application of risk and how it might define therapy in CLL patients. (Fig. 1).You start with a FISH panel and determine the IGHV mutation status. From there, you move into the mutated or lower-risk versus unmutated or higher-risk category. If lower risk, the treatment (if indicated) will typically begin with traditional chemoimmunotherapy. If higher risk, the patient will likely receive the relatively new Bruton tyrosine kinase, or BTK, inhibitors. Now there are also other small-molecule inhibitors available, such as the PI3Kα and BCL2 inhibitors, for the small percentage of patients who might be intolerant of the BTK inhibitor or whose disease progresses.
Pathologists need to be aware of patients with (del)17p and/or TP53 mutations. Both are associated with poor outcomes and relatively resistant to standard chemotherapy and chemoimmunotherapy regimens. Patients with these mutations fare much better when treated with small-molecule inhibitors of BTK, phosphatidylinositol 3-kinase, or BCL2. Progression-free survival and overall survival of CLL patients with a (del)17p or a TP53 mutation but without (del)17p are similar. Both (del)17p by FISH and TP53 mutation by sequencing have prognostic and predictive value and should guide therapeutic decisions.
In our laboratory, where we do a lot of TP53 mutation analyses, the test is looking at exons four to nine, which covers more than 90 percent of the described pathologic mutations. The key is that sufficient clonal B-cells—at least 25 percent—are needed to get the analytical sensitivity out of the assay. We do it by Sanger sequencing, and we do a pre-sort of the peripheral blood clonal B-cells in advance to enrich for relatively pure CD19+ B-cells, so we can be sure we have the right mix of cells to do the assay correctly.
Let’s talk about the antibody structure and function of IGHV because it’s important to understand how recombination occurs and what is meant by variable mutations. Antibodies are composed of a Fab region (variable fragment antigen binding) and an Fc region (constant fragment crystallizable). The Fab region is composed of one heavy and one light chain, and each of those chains has one variable region and one constant region. CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
