CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Children’s Mercy Kansas City. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Laurel M. Bowen, MD
Eugenio Taboada, MD
Linda D. Cooley, MD, MBA
Alan S. Gamis, MD, MPH
Midhat S. Farooqi, MD, PhD
January 2021—Central nervous system high-grade neuroepithelial tumor with BCOR alteration (CNS HGNET-BCOR) is a rare and genetically distinct CNS neoplasm that occurs predominantly in the pediatric population. Previously, these tumors had been categorized as primitive neuroectodermal tumors of the central nervous system (CNS-PNET).1 Sequencing of these tumors reveals characteristic in-frame internal tandem duplications (ITDs) in exon 15 of the BCOR gene, cytogenetic location Xp11.4. BCOR ITDs are not unique to CNS HGNET-BCOR and were first described in pediatric clear cell sarcomas of the kidney (CCSK).2 They have since been identified in other rare pediatric tumors, including primitive myxoid mesenchymal tumor of infancy (PMMTI) and undifferentiated round cell sarcoma of infancy (URCSI), as well as in adults with high-grade endometrial stromal sarcomas (ESS).3-5 Current data show that these tumors are generally associated with poor progression-free and overall survival.
Case report. A previously healthy and ambulating 18-month-old female presented to our institution with a history of progressive ataxia over the prior few weeks. A large well-demarcated mass (5.6 × 6.0 × 6.4 cm) in the midline cerebellum was detected on MRI with no metastases identified (Fig. 1A). The patient underwent a posterior fossa craniotomy with gross total resection. Histologic examination showed poorly differentiated tumor cells with high nuclear-to-cytoplasmic ratios and round to oval and irregular hyperchromatic and vacuolated nuclei arranged in a reticular architecture with scattered microcystic spaces and a prominent myxoid background (Fig. 1B–C). Patchy areas of hemorrhage and necrosis along with scattered microcalcifications were present. There were frequent mitotic figures (22 per 10 high-power fields), and the Ki-67 proliferative index was high at approximately 60 percent. The tumor cells showed neuroepithelial differentiation with immunoreactivity for synaptophysin and NeuN (Fig. 1D–E). The tumor cells were also immunoreactive for GAB1 (cytoplasmic) and YAP1 (nuclear and cytoplasmic), with only cytoplasmic beta-catenin immunoreactivity, providing evidence of SHH (Sonic Hedgehog) signaling pathway activation via immunohistochemistry. GFAP highlighted scattered entrapped glial cells. No malignant cells were seen on cytological examination of the cerebrospinal fluid.
As a diagnosis of HGNET was suspected, Sanger sequencing of exon 15 of the BCOR gene was performed on DNA isolated from fresh frozen tumor tissue. An in-frame internal tandem duplication in exon 15 of the BCOR gene, c.4997_5110dup (p.Leu1703_Gly1704ins38), was detected (Fig. 2A–C). Additional testing included microarray analysis, which showed loss of the entire chromosome 3 long arm, and chromosome analysis (Fig. 2D), which showed an unbalanced translocation involving chromosome 3 and 21 with loss of 3q (46,XX,der(3;21)(q10;q10)[6]; 46,XX[14]). This karyotypic abnormality was not a known recurrent aberration associated with any particular tumor type. Additional reference laboratory testing showed the tumor cells had strong diffuse nuclear immunoreactivity for BCOR. BCOR rearrangement was not detected by fluorescence in situ hybridization testing. However, approximately three percent of the nuclei showed three BCOR signals, consistent with a duplication.
A diagnosis of high-grade neuroepithelial tumor with BCOR alteration was rendered. The patient was treated with intensive chemotherapy with autologous stem cell rescue per the Children’s Oncology Group ACNS0334 protocol, regimen A, allowing the patient to avoid methotrexate exposure. Radiation was deferred due to the significant neurocognitive complications it may cause in patients under 36 months of age. She completed the treatment on schedule over approximately six months. The patient was well and free of disease for six months off therapy, but then had tumor recurrence localized to the primary tumor bed without evidence of other site involvement. She then underwent an additional three cycles of intensive monthly chemotherapy and was determined to be in second complete remission afterward. She remains well and disease free to date at three years and two months of age.
Discussion. CNS HGNET-BCOR is a rare tumor, predominantly occurring in young patients, that demonstrates aggressive behavior. In a series of 10 patients followed in the initial study describing this entity, median progression-free survival was 12 months and median overall survival was 24 months.1 Optimal treatment protocols have yet to be defined for CNS HGNET-BCOR. This patient was treated per established protocols for so-called CNS PNETs for patients under 36 months of age. Previously, PNETs and medulloblastomas were treated similarly; however, it was found that patients with PNETs showed no benefit from the addition of methotrexate. Therefore, she received intensive chemotherapy with autologous stem cell rescue (Children’s Oncology Group ACNS0334 protocol, regimen A) without methotrexate. Due to her age, radiation was deferred. She was disease free for six months off therapy, after having completed six months of therapy, but then had tumor recurrence. Additional chemotherapy induced a second complete remission. Longer follow-up will be needed to assess the efficacy of this treatment. Of note, this patient’s tumor showed evidence of SHH pathway activation via immunohistochemistry, which has previously been reported in this tumor type, and offers another potential route for therapy with agents such as vismodegib, itraconazole, and arsenic trioxide.6
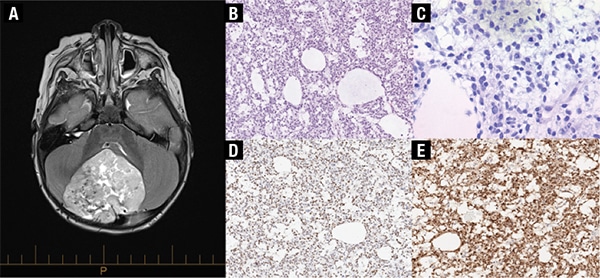
Fig. 1. A) MRI demonstrates a 5.6 × 6.0 × 6.4 cm mass in the cerebellum, mildly eccentric to the right of midline with heterogeneous signal intensity. B) H&E, 160×, and C) 400×. The tumor consists of mildly pleomorphic cells with round to oval nuclei and a small amount of pale eosinophilic to clear cytoplasm. There are microcystic changes and a myxoid background with increased mitotic activity. D) The tumor cells show immunoreactivity for synaptophysin, and E) NeuN indicative of neuroepithelial differentiation (160×).
This diagnosis should be considered in CNS tumors displaying histologic features of relatively uniform oval and elongated cells with fine chromatin and eosinophilic or clear cytoplasm, perivascular pseudorosettes, fibrillary processes typical of glial differentiation, a rich arborizing capillary network, increased mitotic figures, necrosis, microvascular proliferation, and microcystic changes. Immunohistochemistry for BCOR can help support the diagnosis and has shown diffuse nuclear reactivity in many previously reported cases of HGNET-BCOR.3,6,7 Likewise, nuclear immunoreactivity for BCOR has also been described in other BCOR ITD-positive tumors including CCSK, PMMTI, URCSI, and ESS.3,8-10 BCOR (clone C10) immunoreactivity has been studied in other normal tissues and various soft tissue tumors without BCOR abnormalities. BCOR immunoreactivity was found in normal testis, but has not been reported in normal brain. Immunoreactivity was also seen in a large proportion of synovial sarcomas and rare rhabdomyosarcomas and myxofibrosarcomas.7,9 However, BCOR immunoreactivity in other CNS tumors is currently unknown, and its expression alone cannot be used to confirm the diagnosis of HGNET-BCOR at present.
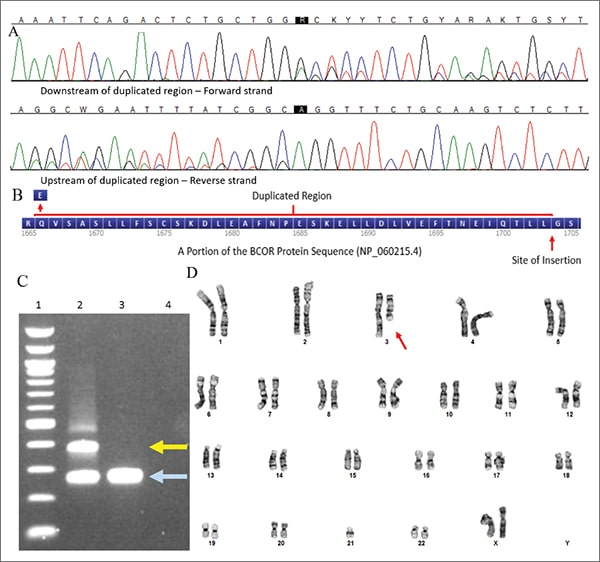
Fig. 2. A) Sanger sequencing detected an in-frame internal tandem duplication in exon 15 of the BCOR gene. Nucleotide labels as called by the software (top) followed by the sequence traces (bottom) are shown for both the forward and reverse strand. B) A protein diagram showing the duplicated region. C) This duplication was also visible via PCR amplification of the region and gel electrophoresis. Lane 1: 100 base pairs ladder; Lane 2: Patient; Lane 3: Negative control; Lane 4: No template control, allele with duplication (yellow arrow), wild-type allele (light blue arrow). D) Karyotype analysis showed an unbalanced translocation involving chromosome 3 and 21 (red arrow) with loss of 3q (46,XX,der(3;21)(q10;q10)).
Molecular testing is necessary to make an accurate diagnosis in suspected cases of HGNET-BCOR. Molecular techniques able to detect BCOR alterations include fragment analysis, Sanger sequencing, next-generation sequencing, FISH, and RNA sequencing.1 A clinically available NGS solid tumor gene panel, which included BCOR as a target gene, would be expected to pick up the relevant duplications in suspected HGNET-BCOR cases. However, care should be taken to select an NGS assay with known ability to detect larger (greater than 50 base pairs) deletions and duplications and to assess DNA for quality as use of heavily degraded DNA would potentially impact sensitivity of detection. While optimal treatment protocols have yet to be defined, proper classification will facilitate choice of treatment and ensure meaningful clinical trials going forward.
Methods. Routine H&E stains and immunohistochemical stains at our institution were performed on 4-µm thick sections from formalin-fixed, paraffin-embedded tissue. DNA was isolated from fresh frozen tissue (tumor cells estimated to be greater than 50 percent of specimen via examination of an adjacent section) per manufacturer’s instructions using a Maxwell Rapid Sample Concentrator instrument and tissue extraction kit (Promega, Madison, Wis.). Purified DNA was subjected to PCR, then bidirectional Sanger sequencing (BigDye Terminator v3.1 Cycle Sequencing Kit and Applied Biosystems 3500 Genetic Analyzer, Thermo Fisher Scientific, Waltham, Mass.) using primers targeting BCOR exon 15 with sequences as previously described.4 Generated sequences were aligned to reference sequence NM_017745.5 and analyzed using Sequencher software (v.5.4.6, GeneCodes, Ann Arbor, Mich.). Other regions of the gene or genome were not sequenced. Variants found were interpreted for clinical significance per the AMP/ASCO/CAP guidelines for somatic variant interpretation.11
Of note, the variant reported above is described per Human Genome Variation Society (HGVS) guidelines. It corresponds to the one reported by Yoshida, et al., and Roy, et al., as: c.5099_5212dup (p.Leu1737_Gly1738ins38) using BCOR reference transcript NM_001123385.1.3,12 References to BCOR exon 16 also exist in the research literature due to use of custom exon numbering; this exon corresponds to exon 15 above (systematic numbering approach).
- Sturm D, Orr BA, Toprak UH, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164(5):1060–1072.
- Ueno-Yokohata H, Okita H, Nakasato K, et al. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet. 2015;47(8):861–863.
- Yoshida Y, Nobusawa S, Nakata S, et al. CNS high-grade neuroepithelial tumor with BCOR internal tandem duplication: a comparison with its counterparts in the kidney and soft tissue. Brain Pathol. 2018;28(5):710–720.
- Kao YC, Sung YS, Zhang L, et al. Recurrent BCOR internal tandem duplication and YWHAE-NUTM2B fusions in soft tissue undifferentiated round cell sarcoma of infancy: overlapping genetic features with clear cell sarcoma of kidney. Am J Surg Pathol. 2016;40(8):1009–1020.
- Juckett LT, Lin DI, Madison R, Ross JS, Schrock AB, Ali S. A pan-cancer landscape analysis reveals a subset of endometrial stromal and pediatric tumors defined by internal tandem duplications of BCOR. Oncology. 2019;96(2):101–109.
- Appay R, Macagno N, Padovani L, et al. HGNET-BCOR tumors of the cerebellum: clinicopathologic and molecular characterization of 3 cases. Am J Surg Pathol. 2017;41(9):1254–1260.
- Haberler C, Reiniger L, Rajnai H, et al. Case of the month 1-2019: CNS high-grade neuroepithelial tumor with BCOR alteration. Clin Neuropathol. 2019;38(1):4–7.
- Santiago T, Clay MR, Allen SJ, Orr BA. Recurrent BCOR internal tandem duplication and BCOR or BCL6 expression distinguish primitive myxoid mesenchymal tumor of infancy from congenital infantile fibrosarcoma. Mod Pathol. 2017;30(6):884–891.
- Kao YC, Sung YS, Zhang L, et al. BCOR overexpression is a highly sensitive marker in round cell sarcomas with BCOR genetic abnormalities. Am J Surg Pathol. 2016;40(12):1670–1678.
- Chiang S, Lee CH, Stewart CJR, et al. BCOR is a robust diagnostic immunohistochemical marker of genetically diverse high-grade endometrial stromal sarcoma, including tumors exhibiting variant morphology. Mod Pathol. 2017;30(9):1251–1261.
- Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4–23.
- Roy A, Kumar V, Zorman B, et al. Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun. 2015;6:8891.
Dr. Bowen is a pediatric pathology fellow, Department of Pathology and Laboratory Medicine, Children’s Mercy Kansas City. Dr. Taboada is chair, Department of Pathology and Laboratory Medicine; pathologist-in-chief and laboratory director; professor of pathology, University of Missouri-Kansas City School of Medicine; and clinical associate professor of pathology, University of Kansas School of Medicine. Dr. Cooley is director of the Division of Cytogenetics; director, clinical genetics and genomics laboratories, and professor of pathology, University of Missouri-Kansas City School of Medicine. Dr. Gamis is associate division director, section of oncology; professor of pediatrics, University of Missouri-Kansas City School of Medicine; and clinical professor of pediatrics, University of Kansas School of Medicine. Dr. Farooqi is director of molecular oncology, Center for Pediatric Genomic Medicine, Department of Pathology and Laboratory Medicine, University of Kansas Medical Center; clinical assistant professor, University of Kansas School of Medicine; and assistant professor, University of Missouri-Kansas City School of Medicine.
Test yourself
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
