April 2024—The rise in fungal infections in recent years troubles Sean Zhang, MD, PhD, for reasons near and far. It’s readily apparent in the patient populations at Johns Hopkins Hospital, where he is director of the mycology laboratory. Especially concerning is the increase in Candida auris following the height of the COVID-19 pandemic, both in terms of colonization and infection cases, says Dr. Zhang, who is also associate professor of pathology, Division of Medical Microbiology, Department of Pathology, Johns Hopkins University School of Medicine. “Since 2022, we suddenly saw an uptick in Candida auris cases across the Johns Hopkins Health System.” But the situation isn’t unique to Johns Hopkins. Pointing to CDC figures, he notes that the tide is rising more broadly as well. The agency reports that in 2020, there were 757 clinical cases and 1,310 screening cases of C. auris in the United States. In 2022, there were 2,377 clinical cases and 5,754 screening cases.
Read More »Subspecialties
Hybrid practice model beckons as solution
April 2024—With the technology now available, could and should remote diagnostic pathology, or at least a hybrid model, become more the norm in the future? Timothy Craig Allen, MD, JD, and Casey P. Schukow, DO, of Corewell Health in Michigan, in an editorial published recently, say the time for one or the other or both has come.
Read More »Need for speed in solid tumor molecular testing
April 2024—As the call for fast turnaround of genetic testing results in tumor profiling grows louder, the need for rapid, reliable test methods becomes more pressing. Meanwhile, with new genetic biomarkers emerging at a rapid pace, “everything has tipped the balance toward comprehensive next-generation sequencing analysis,” said Maria E. Arcila, MD.
Read More »Microscope to image—big lift but also a blueprint
April 2024—The Food and Drug Administration in February cleared Proscia’s Concentriq AP-Dx digital pathology software for the purpose of primary diagnosis.
Read More »Billing headwinds grow stronger for labs
April 2024—In billing for pathology and laboratory services, the hurdles are only getting higher. Narrow networks, prior authorizations, claims denials. Payers “have deeper pockets and figure they can outlast us,” said Joe Saad, MD, chair of the CAP Council on Government and Professional Affairs, in a Feb. 14 roundtable led online by CAP TODAY publisher Bob McGonnagle. He and others talked about AI, digital pathology codes and molecular Z-Codes, biomarker testing, and unity within the laboratory community.
Read More »How Duke’s molecular diagnostics lab retains and trains
April 2024—Too few people, too much to do. In that, Duke Health’s molecular diagnostics laboratory is no different from any other laboratory. But competing for staff on the basis of money alone is out. “The reality is that in today’s labor market, any molecular technologist can always find a job that pays more,” says Barbara Anderson, PhD, MB(ASCP)CM, analytical specialist in Duke’s molecular diagnostics laboratory, Division of Molecular Pathology, Genetics, and Genomics.
Read More »AMP case report: Use of molecular techniques to solve a challenging case of primary cutaneous marginal zone lymphoma
April 2024—Primary cutaneous marginal zone lymphoma (PCMZL) is a newly recognized, distinctive subtype of non-Hodgkin’s lymphoma. This low-grade lymphoma predominantly presents as papules or nodules within the skin of middle-aged adults. Formerly grouped under the extranodal marginal zone lymphoma (EMZL) category, the World Health Organization’s fifth edition classification of hematolymphoid tumors now recognizes PCMZL as a distinct entity.
Read More »A how-to guide to quality management in clinical labs
CAP Publications released this month its newest book, Quality Management in Clinical Laboratories: Optimizing Patient Care Through Continuous Quality Improvement. It is a second edition; the first was published in 2005. Twenty-one contributors cover everything from laboratory staff and informatics to all phases of testing and the laboratory quality management plan.
Read More »Game’s afoot in bladder cancer research
March 2024—Like identifying the shift in battle that leads to victory, or the battle that wins the war—let alone declaring a war’s ultimate victor—it’s hard to gauge the whens, ifs, and hows that mark progress in medicine. For those who are deeply rooted in bringing advances to testing in urothelial cancers, current research is flourishing and flummoxing. In early and late stage, both for bladder and upper tract disease, recently approved therapies are leading to better outcomes for patients. More immunotherapies and antibody-drug conjugates are on their way, and with them come new options for testing. But as with any cancer, researchers follow numerous promising paths, knowing that some will dead-end and others will succeed primarily (albeit usefully) in raising more questions. Nevertheless, they continue to rally the work forward, with multiple breaches, and Agincourt, ever in sight. For experts such as David McConkey, PhD, progress will best be measured by how regularly precision makes its way into the clinical setting.
Read More »From training to first jobs, can the transition be made easier?
March 2024—Pathology trainees and training programs vary, as do first jobs, but the first year in pathology practice is generally said to be a tough one, largely because of the transition to fully independent case sign-out.
Read More »In diabetes patients, biomarker use for early-stage HF
March 2024—For patients with type 2 diabetes, the cardiac biomarkers are a better predictor of early-stage heart failure than conventional risk prediction scores. “We need to use biomarkers,” says Petr Jarolim, MD, PhD.
Read More »Survey probes staff shortage in genomics labs
March 2024—From a technologist workforce perspective, clinical genomics laboratories are in trouble. “It’s truly a crisis,” said Marco Leung, PhD, clinical director of the Steve and Cindy Rasmussen Institute for Genomic Medicine at Nationwide Children’s Hospital in Columbus, Ohio.
Read More »AP and CP reporting, from interfaces to IT wishes
March 2024—Anatomic and clinical pathology reporting—what’s working, what’s missing. Three pathologists (all board certified in informatics) and representatives of three information system companies met online Dec. 19 with CAP TODAY publisher Bob McGonnagle to talk about reporting needs and what’s optimal. The first half of their discussion was published in the February issue, with CAP TODAY’s guide to anatomic pathology computer systems. The second half begins here.
Read More »What’s going on? Interpreting urine toxicology cases
March 2024—For urine toxicology screening, immunoassays are automated and rapid but have variable sensitivity and specificity and results are considered presumptive. Mass spectrometry, used for confirmation, has superior sensitivity and specificity but is labor-intensive and slow and requires significant expertise.
Read More »AMP case report: Acute myeloid leukemia with hyperdiploidy
March 2024—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. This month's report comes from Aga Khan University in Karachi, Pakistan. Case. An 87-year-old male with a clinical history of hypertension and sick sinus syndrome presented with a one-month history of fever, generalized weakness, and weight loss. There was no lymphadenopathy or hepatosplenomegaly on physical examination. Bone marrow examination was performed to evaluate for cytopenias.
Read More »In urinalysis, compromises, collections, and rules
March 2024—Reflex criteria, middleware, bladder cancer screening, point of care, controls, and collections came up in CAP TODAY’s Jan. 16 roundtable on urinalysis. Six people weighed in, with CAP TODAY publisher Bob McGonnagle leading. Their take on where things stand and where they can be better follows. CAP TODAY’s guide to urinalysis instrumentation begins here. Tim Skelton, in last year’s ...
Read More »Doing more, doing better in bladder cancer
February 2024—From her vantage at the University of Texas MD Anderson Cancer Center, Donna Hansel, MD, PhD, has a clear view of cancer’s latest frontiers. Progress and breakthroughs are the norm. But even she sounds impressed when she surveys the changes in her specialty, urothelial cancer. “We are now thinking what we never before thought was possible: We are thinking about cures and lifelong remission from disease,” says Dr. Hansel, division head and professor of pathology and laboratory medicine. It’s been a long time coming, says Dr. Hansel, who is also the Dr. Eva Lotzova and Peter Lotz memorial research chair. The disease historically has been caught in a sort of prepositional triangle—underfunded, overlooked, and underdiagnosed—with serious consequences. For years, she says, “We thought bladder cancer had only one treatment”—BCG, or Bacillus Calmette-Guérin, therapy. Because the field lacked a large volume of research to propel better diagnostics and treatments, “people died of this disease because it progressed.”
Read More »As AI use expands, ethics at the leading edge
February 2024—Artificial intelligence is sizzling, so much so that New Yorker magazine, evoking the dazzling and the potentially devouring nature of AI technology, tagged 2023 as “The Year A.I. Ate the Internet.”
Read More »Biomarker tests with discrepant results—why the differences?
February 2024—When multimodality testing reveals discordant biomarker results, which method is correct? Annette S. Kim, MD, PhD, and JinJuan Yao, MD, PhD, in a CAP23 session last fall used their cases to share strategies for resolving discrepancies—or, in some cases, what look like discrepancies.
Read More »With pipeline for pathologists, others lacking, eyes on AI
February 2024—Artificial intelligence and Medicare Advantage contracts were at the center of the Jan. 2 Compass Group virtual roundtable led by CAP TODAY publisher Bob McGonnagle. “If you want to get into the AI world, there are many lanes you can swim in,” said Michael Feldman, MD, PhD, of Indiana University School of Medicine.
Read More »The race to keep pace with drug use changes
February 2024—Xylazine prevalence, lab-developed testing, and new technology are converging at Yale New Haven Health in a way that gives rise to questions, worry, and new hope for faster drug testing.
Read More »AP and CP reporting—the needs, the caveats
February 2024—Anatomic and clinical pathology reporting—what’s working, what’s missing. Three pathologists (all board certified in informatics) and representatives of three information system companies met online Dec. 19 with CAP TODAY publisher Bob McGonnagle to talk about reporting needs, the changes, what’s optimal. The first half of their discussion begins here; the second half will be published in the March issue.
Read More »More progress, fewer barriers for PGx testing
January 2024—Sometimes even superb ideas can also turn out to be quite, well, bothersome. Zoom meetings. Bridal showers. Bike lanes. Parking apps. QR menu codes. And—if laboratories aren’t careful—the same can be true of pharmacogenomic testing. Just ask Ann Moyer, MD, PhD, associate professor, laboratory medicine and pathology, Mayo Clinic. When it comes to pharmacogenomic testing, laboratory medicine brings significant expertise to the table. But in clinical settings, physicians who prescribe the medications need to be familiar with how to use the test results. They also need to work with the lab to decide which tests, for which genes or gene-drug pairs, will be most helpful for their patients, she says. “Especially if you’re going to start incorporating clinical support alerts into the EHR,” adds Dr. Moyer, who was chair of (until Dec. 31; she is now advisor to) the CAP/ACMG Biochemical and Molecular Genetics Committee. “If the practice doesn’t actually want them, then you’re just going to end up annoying them.”
Read More »The who, when, and why of thrombophilia testing
January 2024—Thrombophilia testing has been shown to be performed far more often than indicated in thromboembolic events, at significant cost to the patient and hospital.
Read More »Lab-developed test proposal reflections and predictions
January 2024—The Food and Drug Administration’s proposed rule on laboratory-developed tests would phase out its existing enforcement discretion approach for oversight of LDTs. Instead, the FDA would classify in vitro diagnostics offered as LDTs as class I, II, or III medical devices depending on their risk to patients.
Read More »A scan of studies on HER2-low breast cancer scoring
January 2024—Much has been said and written about scoring HER2-low breast cancer, and it has its difficulties. But there are steps and tools to support scoring, and Savitri Krishnamurthy, MD, last fall shined a light on them and several HER2-low breast cancer-related studies.
Read More »7 pointers for POC cardiac troponin measurement
January 2024—Seven recommendations for the use of cardiac troponin measurement at the point of care were published last year and reported in a session at the Association for Diagnostics and Laboratory Medicine annual meeting, shortly after the recommendations appeared in print.
Panelists on viscoelastic and other coag assays
January 2024—Viscoelastic assays and other coagulation tests were front and center when CAP TODAY publisher Bob McGonnagle on Nov. 20 convened seven people in an online roundtable. Oksana Volod, MD, and Eric Salazar, MD, PhD, and five company representatives weighed in on, among other things, appropriate test use, automation, and laboratory-developed tests. What they said begins here.
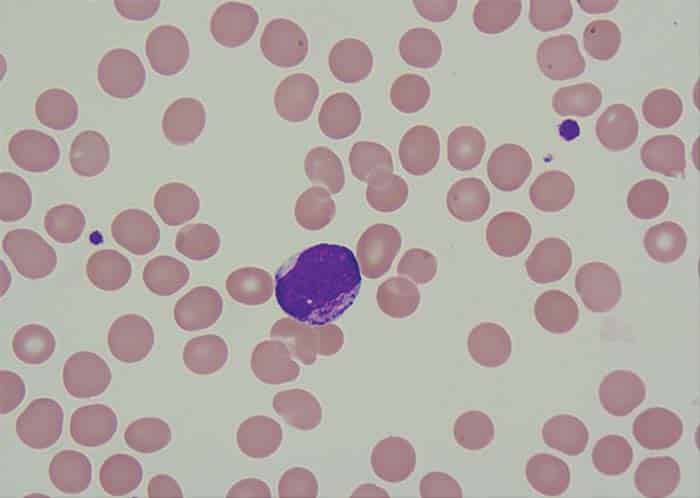
Read More »And the band neutrophil counts play on
December 2023—The recent CAP proficiency testing questionnaire was meant to be the coup de grâce. Hematology PT participants were asked about their band neutrophil reporting practices and, given that these manually generated counts were supposedly on their way out decades ago, the authors of the survey questionnaire expected to see very little activity. The survey, they hoped, would be a way to pound the final, data-driven nail in the coffin. Or, as lead author Maria (Ria) Vergara-Lluri, MD, puts it, “We thought this had all been laid to rest 30 years ago.” It wasn’t. Says Dr. Vergara-Lluri: “Surprise: 86 percent of labs that participated still report bands.” The results of the survey upended many of the assumptions, if not hopes, the authors might have had. Among laboratories that reported manual differentials, they found that most reported bands (4,554 of 5,268). Moreover, only 73 percent reported band reference ranges. On the morphologic challenge, bands classified as “easy” were indeed easy—participants classified them well.
Read More »In fee schedule final rule, lower cuts than proposed
December 2023—In the 2024 Medicare physician fee schedule final rule, the Centers for Medicare and Medicaid Services reacted favorably to the CAP’s advocacy to mitigate payment decreases to pathologists next year. Overall, payments to pathologists are expected to decrease by an estimated 2.7 percent.
Read More »New guidance on lab analysis in diabetes
December 2023—The third and latest edition of recommendations for laboratory analysis in diagnosing and managing diabetes mellitus, released this summer, provide guidance on, among other things, ketone testing, glycolysis, and point-of-care testing. The last such recommendations were published in 2011.
Read More »In ED/urgent cares, the lab tests and the POC team
December 2023—A point-of-care testing team from TriCore was part of standing up three dual emergency department/urgent care centers in as many years, with a fourth set to open in March 2024. “They are super busy, as was expected. There’s a great need for this type of site,” says Kathleen David, MT(ASCP).
Savings follow allergy, autoimmune test consolidation
December 2023—Hoi-Ying Elsie Yu, PhD, D(ABCC), isn’t new to workflow optimization. As system director of chemistry, point-of-care testing, and preanalytics for Geisinger Medical Center in Danville, Pa., for the past decade, she has undertaken initiatives to maximize efficiency in complicated parts of the laboratory whenever she can.
Read More »Digital pathology and AI—drivers, budgets, and jobs
December 2023—Digital pathology and AI—the push, the potential, the changing questions, the reimbursement, and the caution. All that and more came up when CAP TODAY publisher Bob McGonnagle on Oct. 17 led a conversation online with pathologists and industry representatives.
Read More »Phlebotomy program gives lift to lab, community
December 2023—The clinical laboratory at Children’s Hospital of Philadelphia is solving two problems at once: its phlebotomist staffing shortage and the need for some in its community to learn a new skill and obtain employment.
Read More »LDT proposal on the radar—little detail, clarity needed
December 2023—For most, laboratory staffing woes continue, despite some letup post-pandemic. CAP TODAY publisher Bob McGonnagle on Nov. 7 got a sampling of where staffing stands as the year end approaches, in his conversation online with members of the Compass Group, an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies. But first a few words from them about the Food and Drug Administration’s proposed rule on laboratory-developed tests.
Read More »AMP case report: Potential von Hippel-Lindau syndrome in a patient with negative germline testing
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Washington University School of Medicine in St. Louis. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Test adds twists to lung disease diagnosis
November 2023—It was a mystery, wrapped less in an enigma than a few layers of bafflement, surprise, and mild irritation. Call it the Case of the Split Lung Specimens. The first hint something was amiss came when Alain Borczuk, MD, vice chair of anatomic pathology and co-director of thoracic pathology, Northwell Health, noticed that he and his colleagues were receiving more insufficient bronchoscopy specimens than usual. “When I say ‘increasing’—we don’t get that many bronchoscopies. It’s not like colon polyps,” says Dr. Borczuk, who is also director of oncologic pathology, Northwell Health Cancer Institute. Normally they would get a handful a week, some of them straightforward cancer cases, although these additional cases were tied to noncancerous conditions. And then the plot thickened even further, with missing pieces—literally. Though no guideline clearly states what constitutes an adequate specimen, Dr. Borczuk says, the samples he and his colleagues were seeing fell markedly short.
Read More »New guidance in checklist on AMR and mass spec
November 2023—In the 2023 edition of the CAP accreditation program checklists is new guidance on analytical measurement range verification and new and revised requirements for mass spectrometry.
Read More »Point-of-care testing scorecard spotlights hits and misses
November 2023—At the point of care, there are testing wins, some losses, and plenty of pitfalls. “Point-of-care coordinators all have the same problems,” says Meaghan Gladstone, applications consultant at Werfen.
In some settings, alternatives to HbA1c acceptable
November 2023—Glycated albumin and fructosamine are highly specific, with high levels suggesting hyperglycemia. This points to their utility in monitoring glycemic control in people with diabetes. “They’re quite useful in the setting of overt hyperglycemia,” said Elizabeth Selvin, PhD, MPH, at this year’s meeting of the Association for Diagnostics and Laboratory Medicine.
Read More »Generative AI, from education to corner cases
Generative artificial intelligence—what it is, how it can be used in pathology, what stands in its way, why the excitement. CAP TODAY publisher Bob McGonnagle spoke about that and more with pathologists Bobbi Pritt, MD, MSc, and Scott Anderson, MD; Ajit Singh, PhD, of Stanford and Artiman Ventures; and Devon Snedden, a health care consultant in artificial intelligence. “There are a lot of excellent possibilities that we’re just starting to understand and explore for the field of pathology,” said Dr. Pritt of Mayo Clinic.
Read More »AI-driven spatial biology: the next next-gen sequencing
November 2023—Spatial biology may be an emerging field, but Kenneth Bloom, MD, says he and other pathologists have been doing it “since we got the microscope.” And he argues it’s going to become “the new, most important lens we look through.” The reason is the emergence of new cancer treatments like immunotherapy and, most importantly, antibody drug conjugates like Enhertu, says Dr. Bloom, head of pathology for Nucleai, a company specializing in AI-powered spatial biology.
Read More »Minds shift on digital path, ‘massive change’ predicted
Is digital pathology on the move? Two who know it well say it is. Esther Abels, a precision medicine and biomedical regulatory health science expert who is CEO of SolarisRTC and former president of the Digital Pathology Association, and Michael Rivers, vice president/lifecycle leader of digital pathology at Roche Tissue Diagnostics, spoke in September with CAP TODAY publisher Bob McGonnagle, who got their take on where things stand.
Read More »Pathology navigators bring molecular test efficiencies
November 2023—Few things in the laboratory can do so much at once: boost histotechnologist productivity, safeguard tissue, offer a career path and help retain staff, keep watch on test utilization, and reduce the number of calls to pathologists and turnaround time, all while advocating for the patient.
Read More »Reports revisited—panel on preferences and pain points
November 2023—Reports—integrated or otherwise—were up for discussion when CAP TODAY publisher Bob McGonnagle convened online in October a group of informatics experts, who spoke of the need for simplicity in a time of growing complexity, ease of access, where Epic isn’t strong. The full conversation follows.
Read More »Digital path’s star rises from the mists
October 2023—In living up to its promise as a new technology that will revolutionize clinical care through greater ease, speed, and accuracy of diagnosis, digital pathology has been sluggish. While many analysts, starting at least two decades ago, forecasted that digital pathology would elbow aside glass slides for good, that milestone is still far out of reach. As health economist and chief executive officer of the New York City-based digital pathology company Paige, Andy Moye, PhD, puts it bluntly: “In probably 90 to 95 percent of the cases in the U.S., a pathologist still makes the diagnosis of cancer the way they did it back in 1910: by looking at a glass slide under a microscope.” Mark Lloyd, PhD, vice president of pathology for Fujifilm, says he wouldn’t be surprised to hear that perhaps only five percent to 10 percent of hospitals have moved beyond using only glass slides to offer pathologists digital pathology capability. In fact, Dr. Lloyd thinks those percentages are overstated. What is the market share for the clinical use of digital pathology?
Read More »Looking ahead to respiratory virus season
October 2023—With respiratory virus season near, those with a close eye on it in August gave the lay of the land for test algorithms, technologies, and forecasts, even as SARS-CoV-2 and RSV cases were rising in parts of the country.
Read More »Soon to be required: current susceptibility testing breakpoints
October 2023—A CAP accreditation program requirement that microbiology laboratories use current antimicrobial susceptibility testing breakpoints, which was added to the checklist in 2021, will go into effect Jan. 1, 2024.
Read More »Inside lab’s experience with assays for Alzheimer’s
October 2023—Orders for cerebrospinal fluid testing for Alzheimer’s disease have grown at Mayo Clinic since spring 2020, when testing was first offered. When aducanumab was approved in May 2021, test orders jumped.
Read More »Fast or comprehensive? Lab offers both for NSCLC
October 2023—For molecular testing in oncology, the choice is often fast or slow. PCR-based platforms are rapid, and comprehensive genomic profiling by next-generation sequencing is slower, and each has its pros and cons.
Read More »New guide to whole blood viscoelastic assays: hemostasis, testing, cases, and applications
October 2023—New this month from CAP Publications is Whole Blood Viscoelastic Assays in Clinical Diagnosis: An Illustrated Case-Based Guide. Viscoelastic testing was designed to determine the cause of intraoperative or trauma-related bleeding to guide hemostatic therapy. CAP TODAY asked the book’s editor, Oksana Volod, MD, about the guide. See her answers and a sample chapter. Dr. Volod is professor of pathology and director of the coagulation consultative service, Cedars-Sinai Medical Center, Los Angeles.
Read More »AMP case report: Identification of multiple germline cancer predisposing gene variants in a single patient during tumor sequencing analysis
October 2023—Next-generation sequencing of tumor tissue has important implications in solid and hematologic malignancies because it can identify genomic variants that provide diagnostic, prognostic, and predictive information to guide clinical management. Variants identified on tumor sequencing can be classified as somatic (acquired after conception) or inherited through germline.
Read More »In hematology, making the most of automated solutions
October 2023—Hematology analyzers and the related workflow, expertise, efficiency, and IT matters were the topic of a roundtable when CAP TODAY publisher Bob McGonnagle met online Aug. 29 with two pathologists and representatives from Horiba, Siemens, Sysmex, CellaVision, Sight, and Abbott. Their conversation follows.
Fernando Chaves, what are the advances in artificial intelligence in the field of hematology, particularly automated hematology, since we spoke during our roundtable at this time last year?
Fernando Chaves, MD, global head of hematology, Siemens Healthineers: Technology now enables full-field digital morphology, a full image of the entire slide scan. Now we can do with hematology what has been done for over a decade in surgical pathology.
Tests for paraneoplastic syndromes in neurology
September 2023—Laboratory testing for paraneoplastic neurologic syndromes is neither commonplace nor cheap. It also comes with its own enigmatical math, as Michael Levy, MD, PhD, recently experienced. As the director of the Neuroimmunology Clinic and Research Laboratory in Massachusetts General Hospital’s Department of Neurology, Dr. Levy keeps an eye on PNS laboratory testing (which is performed at Mayo Clinic) at his institution.
Many knots to untangle in lab test names
September 2023—Ambiguities, inconsistencies, omissions, and other defects in the naming of laboratory tests can send test orders and results interpretation awry, particularly with some of the most common tests. Even among clinicians and laboratorians working at the same hospital for years, smooth sailing is not guaranteed. The authors of a study published in Archives of Pathology & Laboratory Medicine hope to change that. Their aim is to alert patient-facing providers and laboratories to the risks that ambiguous or nonstandardized laboratory test naming poses and to provide practical rules for minimizing those risks.
For infectious disease, what tests and at what time in disease course
September 2023—For the tickborne and mosquito-borne arboviruses, which are frequently more transient in blood and tissue than other pathogens, both molecular and serologic testing have a place in the diagnostic toolkit.
Read More »Lab’s steps to fewer contaminated urine cultures
September 2023—A casual comment made in a routine exchange in an Avera McKennan Hospital laboratory sparked a five-year campaign to bring down the urine culture contamination rate. “I feel like all I do is report contaminated cultures,” a microbiology technologist said in 2016.
Read More »New viscoelastic testing requirement in checklist
September 2023—A proficiency testing accreditation requirement in the new checklist edition was revised to add clarification, and a new requirement on viscoelastic testing will close an existing gap.
Read More »People, partners, and platforms at the point of care
September 2023—Point-of-care testing—the requests and the committees that oversee them, the connectivity, what AI might bring. CAP TODAY publisher Bob McGonnagle on July 21 met online with a laboratory operations director and a medical director from large health systems and with company representatives for a look at where things stand today. Their conversation follows.
Disruptive technologies—what impact on lab workflow?
September 2023—New from CAP Publications is Disruptive Technologies in Clinical Medicine, by Frederick Kiechle, MD, PhD. In his new book Dr. Kiechle says “disruptive technologies offer new paradigms in diagnostic medicine.” Technology-driven disruptions are stimulated by the need to improve patient care, he writes, and they have been “a feature of the practice of clinical pathology since the inception of the first clinical laboratory in 1895 at the University of Pennsylvania, the William Pepper Laboratory.”
Read More »AMP case report: Lung micropapillary adenocarcinomas revisited
September 2023—CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Henry Ford Hospital. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »Genetic counseling within the laboratory: For oncology cases, lab’s consult service plugs gap
September 2023—What happens when an oncologist cannot confidently determine what type of genetic test to order for their patient? Where can a provider turn if they do not know whether a genetic variant is clinically actionable? As genetic testing becomes a more integral part of personalized medicine and health care in general, there is a growing need to bridge the gap between those skilled in molecular diagnostics and those on the patient-facing side of care. In response to this need, the Center for Integrated Diagnostics (CID), a high-complexity molecular diagnostics laboratory at Massachusetts General Hospital, created its Consultation Service.
Read More »Low level limbo in HER2 breast cancer
August 2023—Seemingly channeling the inspiration of Magritte and his famous pipe, pathologists are painting a new picture of what has long been an everyday object in their own world: HER2. To paraphrase the master: Ceci n’est pas facile. For years, HER2 testing in breast cancer has seemed self-evident, ever since the HER2-targeted therapy trastuzumab and its companion diagnostic arrived on the scene a quarter of a century ago. Pathologists became comfortable using immunohistochemistry to identify 3+ cases and turning to in situ hybridization techniques to sort through less obvious ones. But early last summer, a variant of the drug, trastuzumab-deruxtecan (T-DXd), shook up that routine. When researchers presented results from the Destiny-Breast04 study at the 2022 ASCO annual meeting, showing that T-DXd significantly improves survival in so-called HER2-low metastatic breast cancer, attendees responded with a minutes-long standing ovation. They then returned from the meeting like evangelicals from the revival tent.
Read More »For patients, demystifying pathology reports
August 2023—With patient access to pathology reports now common via online portals, the question some have asked is what can be done to make them easier for patients to understand.
Read More »Few but notable— new accreditation checklist changes
August 2023—Climate control, calculation verification, block retention, and histocompatibility section director (technical supervisor) qualifications are among the areas in which laboratories can expect to see revisions in the new edition of the CAP laboratory accreditation checklists, to be released this month.
Read More »Upon viral infection, assessing the host nasal epigenome
August 2023—Analyzing the nasal epigenome can shed light on viral infections, strain differences, and potentially infection severity, and for influenza B in particular the results are striking.
Read More »In anatomic pathology labs, a balancing act
August 2023—Anatomic pathology laboratories—the pressures, the promise of technology to alleviate them, and the seemingly unprecedented rates of change. CAP TODAY publisher Bob McGonnagle gathered pathologists and company representatives online on June 21 to talk about it all. From pathologist coverage to IT, from tumor boards to questions job candidates in pathology should ask, here’s what they told us.
Read More »New cassette printer a standout? Users think so
August 2023—No jams, downtime, or lost time. Plenty of savings, speed, and employee satisfaction. Users of what General Data calls its next-generation cassette printer for histology laboratories say they’ve seen all that and more.
Read More »AMP case report: Small intragenic structural variants in SATB2-associated syndrome
CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Washington University School of Medicine in St. Louis.
Read More »Cytopathology in focus: How to approach cytology of unknown primary
August 2023—We discuss in this article a common problem that all cytopathologists come across frequently in their practice: tumors of unknown primary origin involving body fluids and other sites. Metastatic tumor cells can disseminate and colonize discontinuous secondary body sites.1 Such tumor metastases may be the patient’s initial presenting complaint to a family physician for deep-seated tumor primaries such as ovaries, pancreas, liver, and certain non-obstructive gastrointestinal tumors.
Read More »Cytopathology in focus: Impact of primary HPV testing on cytology lab statistical analysis
August 2023—Cytology gynecologic statistical laboratory data must be analyzed in the context of changing screening modalities and patient populations. This is especially important with the increasing use of primary high-risk HPV screening because diagnostic metrics will be markedly different in that patient population.
Read More »Cytopathology in Focus: Lung cytopathology reporting: WHO system and cases
August 2023—Accurate and timely diagnosis is the cornerstone of effective patient care, particularly in the field of pulmonary pathology. To address the challenges health care professionals face in diagnosing and reporting respiratory conditions, the International Academy of Cytology, together with the International Agency for Research on Cancer, recently developed the World Health Organization Reporting System for Lung Cytopathology
Read More »Turning questions to answers in drug testing
July 2023—As she surveys the opioid epidemic in North America, Christine Snozek, PhD, D(ABCC), could be tempted to think that a ripped-from-the-headlines reality has landed in clinical laboratories as well as on TV crime dramas. With the number of opioid-related deaths increasing in recent years, particularly since the start of the pandemic, drug testing demands have increased for labs as well, says Dr. Snozek, codirector of clinical chemistry and support services and director of point of care and central processing at Mayo Clinic in Arizona. If only she could turn to the entertainment industry for a technology-based solution. “I wish we had CSI lab capabilities,” she says, referring to the long-running police procedural. “You could run a sample and find all the drugs known to man on one test. If they could go ahead and release that technology, that would be wonderful,” jokes Dr. Snozek. Given that laboratories are unlikely to take a meeting with network executives, Dr. Snozek and others in the field will have to look elsewhere.
Read More »For SARS-CoV-2, clearing the air on EUA tests
July 2023—As the COVID-19 federal public health emergency drew to a close in mid-May, industry experts explained what will and won’t change for the laboratory and weighed the fallout from the drop-off in SARS-CoV-2 testing.
The human gut microbiome and blood biochemistry connection
July 2023—The human microbiome has been called the forgotten organ, and at one time it was. But not in the past 10 years. James Versalovic, MD, PhD, made that clear in his talk at the Association for Molecular Pathology meeting last year.
Read More »For sepsis Dx, MDW biomarker brought into the mix
July 2023—When Butler Health System in early 2020 installed the Beckman Coulter DxA 5000 automation line, its hospitals were among the first in the country to do so. At the same time, Butler went live with the DxH 900 hematology analyzer.
Read More »Cytomegalovirus in IBD: where to biopsy, whom to treat
July 2023—Though it’s been suggested that newer drugs have made cytomegalovirus less relevant in patients with inflammatory bowel disease, CMV remains an important opportunistic infection in patients with IBD. Knowing where to biopsy and how many are needed is one of the histologic challenges, said Joseph Misdraji, MD, associate professor of pathology, Yale School of Medicine, in a CAP22 session.
Read More »First a probe purchase, then an academic consortium
July 2023—Bringing new technology into laboratories is important for pathology as a field and for patients—and only getting more difficult. “Each new wave of technology is more complicated than the last,” Jeremy Segal, MD, PhD, said at the USCAP meeting this spring.
Read More »Lab leaders on moving markets and tipping points
July 2023—Digital pathology, the pathology workforce, and the clinical demand for subspecialty expertise were some of what Compass Group lab leaders took on in their June 6 conversation, with CAP TODAY publisher Bob McGonnagle leading the way.
AMP case report: A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia
July 2023—A germline GATA2 c.121C>G (p.P41A) variant in a patient with an unusual acute promyelocytic leukemia CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Emory University School of Medicine. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Read More »In search for Candida auris, labs all in
June 2023—A bad-news, good-news, bad-news, good-news bass line thrums through the ongoing story of Candida auris as it continues to spread in the United States. Initially identified in Japan, in 2009, in an ear specimen—hence the auris—the yeast was first reported in the United States in 2016. Like certain other pathogens, C. auris’ domestic presence appeared to be linked to travel-related cases, then quickly spread, first to the metropolitan regions of Chicago and New York City and now to more than half the states. That’s worrisome. Yet the spread hasn’t been unbridled. Early fears that it would sweep indiscriminately through all patient populations have not been realized. “It’s not as virulent as albicans,” says Sixto M. Leal Jr., MD, PhD, director of the clinical microbiology laboratory and of the fungal reference laboratory, University of Alabama at Birmingham, and a member of the CAP Microbiology Committee. “It’s about as virulent as Candida glabrata. It’s not too much of a significant threat if you’re healthy.”
Lyme algorithms: stick to standard, move to modified?
June 2023—For Lyme disease testing, immunoblots became optional in 2019 when the FDA cleared enzyme immunoassays for use as part of a modified two-tiered testing algorithm. “It was a historic event in the world of Lyme diagnostics,” says Elitza Theel, PhD, D(ABMM).
Reorganize, promote, shift, assess—staying staffed amid a shortage
June 2023—The labor shortage may ease now and then for some laboratories in some areas, but the general outlook is that it will stick around for a while—if not forever.
Read More »Weighing the risks in HIV, HCV algorithmic testing
June 2023—For HIV and HCV algorithmic testing, the workflow options have risks to consider. Molecular testing performed as an automatic reflex on the same sample used for the serologic testing risks carryover contamination, and requiring a dedicated sample for the molecular assay risks incomplete testing.
Read More »Workflow, specimen transport under the lens at AACC
June 2023—Transporting specimens to the laboratory, and processing and distributing them within the lab, will be what AACC meeting-goers hear about in a session next month.
Read More »Kaiser, Geisinger: the first of similar headlines?
June 2023—Consolidation in health care makes the news often. But the coming together of Kaiser Permanente and Geisinger Health and their launch of Risant Health got special attention. CAP TODAY publisher Bob McGonnagle asked Compass Group members for their take on the acquisition when they met online on May 2.
Read More »ABPath’s plans for transparency, competency pilot
June 2023—Three initiatives are underway at the American Board of Pathology, one of which its CEO Gary Procop, MD, MS, describes as “a new era of transparency and collaboration.”
Read More »Appendiceal lesions: features, subtypes, patterns
June 2023—Although goblet cell adenocarcinoma can label for neuroendocrine markers, it behaves as an adenocarcinoma and is staged as such. And it’s important to distinguish goblet cell adenocarcinoma from tubular neuroendocrine tumor, a rare subtype of neuroendocrine tumor.
Read More »The intersection of news, core labs, and lab costs
June 2023—As CAP TODAY assembled its annual guides to chemistry and immunoassay analyzers (for this issue and the July issue), publisher Bob McGonnagle brought together IVD manufacturers and lab leaders to talk about consolidation and ever-larger health systems, technology, efficiencies, and centralized and decentralized testing.
Colorectal cancer next on HER2 horizon
May 2023—Behold the common coin. Note its two sides, its easy flippability. Here is Joseph Pizzolato, MD, with the first coin toss. Given the expanded use of biomarkers with a variety of tumors, and constantly evolving assays, how hard is it for medical oncologists to navigate testing? “It’s not difficult at all now,” says a cheerful Dr. Pizzolato, medical director of the comprehensive therapeutic unit of Sylvester Comprehensive Cancer Center, University of Miami Health System, as well as medical director of the Aventura satellite at Sylvester. With third-party companies integrating test ordering directly into electronic medical records, he adds, “It’s getting even easier to order tests and see the results.” Agreed, says his colleague Rhonda Yantiss, MD, director of surgical pathology, Department of Pathology and Laboratory Medicine, University of Miami Miller School of Medicine. And therein lies the problem. “It’s kind of a mess,” she says. In practice, precision medicine is becoming both more and less precise.
Read More »Alzheimer’s biomarkers: CSF reports, plasma prospects
May 2023—For fluid biomarkers in the diagnosis of Alzheimer’s disease, the pace of research and related industry and regulatory developments in the past year has been brisk.
Read More »‘Hundreds of variants’—Staying alert to HbA1c method interference
May 2023—Of the five methods used to measure HbA1c—immunoassay, boronate affinity, enzymatic, capillary electrophoresis, and ion-exchange HPLC—only the latter two can alert the laboratory and physician to the presence of a suspected hemoglobin variant. Even so, there are reasons to be cautious.
Read More »In-house or send-out? Lab approaches to gene panels
May 2023—Achieving standardization and setting up processes around the use of next-generation sequencing panels for the care of patients with cancer is a long road requiring a lot of expertise.
Read More »Supported? Satisfied? Keeping staff from moving on
May 2023—When hiring is difficult, how to improve retention becomes what it’s all about. Linking sign-on bonuses with performance metrics rather than time in the job is one way to try to retain employees in an industry in which demand for staff far outweighs the supply.
Read More »Appendiceal lesion cases, clues, and cautions
May 2023—How to distinguish appendiceal diverticular disease and appendiceal polyps from mucinous neoplasms was just part of a CAP22 course on appendiceal lesions, led by Maryam Pezhouh, MD, MSc, of the University of California, San Diego, and Jacqueline Birkness-Gartman, MD, of Johns Hopkins University School of Medicine.
Read More »The outlook for in-house next-generation sequencing
May 2023—Bringing next-generation sequencing in-house was at the center of a March 27 roundtable led by CAP TODAY publisher Bob McGonnagle, with costs, reimbursement, equity, and the electronic health record part of the conversation. Jeremy Segal, MD, PhD, of the University of Chicago, explains why the Genomics Organization for Academic Laboratories was formed. “By lowering barriers and encouraging cooperation,” he said, “we’ve seen our labs increase the pace of development and the quality of the assays they’re bringing on.”
Read More »Inside the WHO Reporting System for Pancreaticobiliary Cytopathology
May 2023—Standardized reporting systems have been developed during the past decade for cytopathology of different organ systems including the pancreaticobiliary system. The Papanicolaou Society of Cytopathology (PSC) in 2014 published the first reporting system for pancreaticobiliary cytology. Studies have demonstrated that implementation of the PSC reporting system has significantly reduced the number of “atypical” interpretations and increased the number of specific diagnoses.
Read More »Adequacy in cytopathology: focus on cytology specimen use in molecular testing
May 2023—In the first article in our series on adequacy in cytology, published in January 2023 (bit.ly/3MDNVzr), we summarized current approaches to defining adequacy for the purpose of primary diagnosis in the majority of specimen types encountered routinely in cytology practice. As we saw, while the essence of adequacy is constant across reporting systems, the technical definitions can vary significantly by specimen type.
Read More »Growing pains put gene panels in a pinch
April 2023—After years of excitement and scientific breakthroughs, the use of molecular testing to guide cancer therapeutics finally is coming into its own. Unfortunately, it appears to have landed in the wrong place at the right time. That place is a lonely spot, surrounded by gaps in economics and coverage, as well as knowledge, guidelines, ordering patterns, turnaround times, reporting, and the like. So plentiful are the gaps that, put together, they could form a vast, inhospitable space, a veritable Colorado Plateau, with molecular testing as a majestic, enticing but remote rocky pinnacle in the middle. Think Monument Valley. It’s worth the trek. The evidence in support of genomic profiling continues to grow. Simply put, “Patients with the right markers who get the right drugs do better,” says Neal Lindeman, MD, vice chair, laboratory medicine and molecular pathology, Department of Pathology and Laboratory Medicine, Weill Cornell Medicine/New York Presbyterian Hospital. But as numerous studies are showing, the lag in testing is growing as well.
Read More »Sorting out celiac disease with serologic testing
April 2023—Celiac disease incidence is up and the diagnostic rate is low, and it can be years from onset of symptoms to diagnosis. “It’s a long diagnostic odyssey, and so in the laboratory business, we’re all in to help,” says Annette Taylor, MS, PhD, associate vice president at Labcorp where she is strategic director of pharmacogenomics and scientific director of molecular genetics.
Read More »After negative CT for brain injury, a biomarker gap
April 2023—Traumatic brain injury triage in the emergency department is badly in need of biomarkers—and ones that can change practice. “If biomarkers don’t change practice, they’re a waste of time,” said W. Frank Peacock IV, MD, professor of emergency medicine, vice chair of research, and research director, Department of Emergency Medicine, Baylor College of Medicine.
Read More »Lab leaders on hires, wages, scanners, and storage
April 2023—How is the demand for biomarker tests linked to new oncology drugs playing out in your health system? It is one of several questions laboratory leaders answered in a March 7 Compass Group call led by Stan Schofield, VP and managing principal of the Compass Group and formerly of NorDx/MaineHealth. That and digital pathology and the cost of storage, staffing and wages, the release of results, and the financial implications of the end of the public health emergency were the topics of the day. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Ancillary tests in gynecologic pathology—what and why
April 2023—With serous ovarian carcinomas and other gynecologic tumors, it’s molecular testing that increasingly sets the treatment course. “As pathologists, it’s exciting to us,” said Sadia Sayeed, MD, speaking at CAP22. “We’re learning that the immunostains we’re interpreting have greater implications than we ever thought they could.”
Read More »A start at standardizing neoplastic cellularity assessment
April 2023—Molecular testing guidelines say that the neoplastic cell content of each specimen should be assessed, but there are no formal recommendations or guidelines on how to do the assessment.
Read More »In billing, No Surprises and other complexities
April 2023—Another administrative layer and “up in the air” is how lab billing experts describe what the No Surprises Act requires of laboratories and where things stand. When they met online March 3 with CAP TODAY publisher Bob McGonnagle, they talked about this and digital pathology and the problems of no or slow payments. “Compared with five years ago, the number of denials has increased and turnaround time on full payment on a claim has lengthened significantly,” said Tom Scheanwald of APS Medical Billing.
Read More »New paths through hematologic neoplasms
March 2023—Updated classifications for hematologic neoplasms are here. Let the complications continue. As with other specialties, hematopathology has been absorbing advances gleaned from molecular and genetic data. In some cases, this can tilt diagnosis away from primarily immunophenotypic approaches. It might lead to splits in what was formerly a single entity. On occasion, it might suggest further testing options that could be of value to patients now, or possibly at a date down the road. Or it might just leave pathologists and their clinical colleagues peering at a lack of data, knowing they have to make decisions nonetheless. Two groups—the World Health Organization and the International Consensus Classification—have put forth classifications to help physicians sort through the complexities. The WHO published a beta version of the fifth edition of its Classification of Haematolymphoid Tumours in July 2022.
Read More »Case review reveals latest on overtransfusion
March 2023—A retrospective study of patients who received blood transfusions at 15 community hospitals found that just over half of the patient encounters reviewed could have been managed without the transfusion of at least one component type, and 45 percent could have been managed without any transfusion.
Read More »Spotlight on ancillary tests in endometrial cancer
March 2023—With endometrial carcinomas and other gynecologic tumors, molecular testing matters, and not only at the diagnostic stage. “We’re rapidly evolving,” said Leslie M. Randall, MD, MAS, division director of gynecologic oncology, Virginia Commonwealth University Health, speaking at CAP22.
Read More »Putting in place a molecular panel for pneumonia
March 2023—When it comes to molecular syndromic panels for pneumonia, there’s both upside and downside, says Neil W. Anderson, MD, D(ABMM), associate professor, Department of Pathology and Laboratory Medicine, Washington University School of Medicine in St. Louis.
Read More »Digging deep to drive recruitment and retention
March 2023—All in on staff retention and solving staff shortages, some made worse temporarily by weather. That’s where laboratories were when Compass Group members told CAP TODAY publisher Bob McGonnagle in their Feb. 7 call where hospitals and labs were aiming their efforts. From safety huddles and stay interviews to arranging for overnight stays and incentives, the work to remain sufficiently staffed continues. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Read More »For those who want it easy, blood draws anywhere
March 2023—The need was always there for some. For others, it’s a matter of convenience. “Home phlebotomy as a concierge service” is how Michael Eller describes what went live in 2019 in New York at Northwell Health, where he is assistant vice president of business development for its laboratories. “It was just a matter of using the capacity we already had in the field and hiring appropriately as needed,” he says.
Read More »Close-up on AI-driven assistive tools in pathology
March 2023—Assessing cardiac allograft rejection from endomyocardial biopsy and assigning a differential diagnosis to cancers of unknown origin have been shown to get a boost from AI-driven computational pathology models. So too has identifying subregions of high diagnostic value on whole slide images.
Read More »In urinalysis, reflex algorithms and other efficiencies
March 2023—Urinalysis was at the heart of a Feb. 7 discussion between CAP TODAY publisher Bob McGonnagle; Ron Jackups Jr., MD, PhD, of Washington University School of Medicine; and Jason Anderson of Sysmex America. “There’s a lot of room to explore what the optimal parameters are to use with the best specificity and sensitivity for a reflex to the sediment analysis or the culture,” Anderson said. Here’s what he and Dr. Jackups said about reflex testing, automation, and middleware.
Read More »Breast cancer biomarkers, classic and new
February 2023—Like a thriving expat, Deborah Dillon, MD, is comfortable moving within worlds both old and new. Specifically, as a breast and molecular pathologist at Brigham and Women’s Hospital, she appreciates the biomarkers she and her colleagues grew up with, so to speak, as well as those that are part of a more recently arrived-at scenery. Not everyone finds both worlds equally riveting. “A lot of people are much more interested in, and excited by, new markers,” she says. “When I talk to people from pharma, this is what they want to hear about.” So do many pathologists, oncologists, and patients—new markers and new therapies have a way of updating hopes. Dr. Dillon understands the persistent thrill of the new, why people want her to talk the language of PIK3CA, PARP inhibitors, MMR, NTRK fusions, ESR1, and the like. But as an in-demand speaker as well as in a recent interview with CAP TODAY, she also advocates for making the old—the longstanding trinity of ER, PR, and HER2—seem new again.
Read More »Getting syphilis testing right as case rates rise
February 2023—Traditional algorithm? Or reverse? Elitza Theel, PhD, D(ABMM), of Mayo Clinic, in an AACC session last year walked through the two primary diagnostic algorithms for syphilis, explaining where the complexities lie and how her laboratory uncovered inappropriate testing for neurosyphilis.
Read More »In surgical services, stewardship steps reduce lab orders
February 2023—Consensus on the best ways to stem unnecessary laboratory testing, and spare health care systems and patients its negative effects, is still elusive.
Read More »Views on point of care versus core and more
February 2023—Point of care or core lab? An old question but a new conversation, this one on Jan. 12 between Stan Schofield, Brian Durkin, and CAP TODAY publisher Bob McGonnagle (asking the questions). Here’s what they said about that and health care economics, autoimmune testing, tube supplies—and, of course, the labor shortage because it affects nearly everything in health care.
Read More »Too high or low? Too long? Lessons in ergonomics
February 2023—Workspace design seldom supports the tasks workers perform, and pain and productivity loss can be the outcomes. Eliminating the risks at the design stage is therefore the best approach. When that’s not possible, retrofitting to reduce or eliminate some (but not all) of the hazards is an option, though it’s difficult and can be costly, says Marissa Pentico, MS, OT/L, CPE, ergonomics coordinator in the Duke University Occupational and Environmental Safety Office.
Read More »Study: Cardiac biomarkers in transgender people
February 2023—Sex hormones, rather than sex assigned at birth, may be a stronger driver of the observed concentration differences between healthy men and women for biomarkers of cardiac disease, say the authors of a study published in JAMA Cardiology (Greene DN, et al. JAMA Cardiol. 2022;7[11]:1170–1174).
Read More »‘Doing more for less and with less’: Turning to IT
February 2023—As this year’s guide to anatomic pathology computer systems was taking shape, CAP TODAY publisher Bob McGonnagle met online with representatives of five companies and with John Sinard, MD, PhD, of Yale University School of Medicine. They talked about the cloud, CPT codes, training of pathology informaticians, and artificial intelligence, for which the time frame in pathology is far longer than it’s been portrayed, in Dr. Sinard’s view. “It will start to impact the careers of some of our trainees, but it’s probably a 10- to 20-year time frame before it plays a major role,” he said. The view of Joe Nollar of Xifin: “Speculation that AI will someday replace pathologists is completely overblown,” though it will help to triage cases and mitigate risk. Their full conversation, which took place Dec. 20, 2022, follows.
Read More »Against all odds, a histology lab in rural Ghana
February 2023—I was fortunate last summer to be part of a team established to build a histology laboratory in rural Ghana. Despite our many challenges, we succeeded in building a laboratory where specimens can now be processed for pathological interpretation. Although many obstacles remain, this laboratory offers great potential to improve the pathology services provided to a large portion of rural Ghana.
Read More »Evaluating post-treatment breast specimens
January 2023—Laura Esserman, MD, MBA, can still recall her Eureka moment. She had just seen a talk on residual cancer burden by pathologist W. Fraser Symmans, MB.ChB, a pioneer in the field. “When I saw Fraser present this,” says Dr. Esserman, director, University of California San Francisco Breast Care Center, “I knew immediately that MRI would work and that residual cancer burden would complement it. MRI was basically a snapshot of RCB over time. I realized that we had to institute RCB—we had to standardize our approach.” Until then, she and her colleagues across the I-SPY trial sites relied on individual pathologist assessment for each case. The pathologic complete response rate, or pCR, hovered at about 34 percent. That insight was soon followed by another. Intrigued by what she heard, Dr. Esserman and her pathologist colleagues from all the I-SPY sites traveled to MD Anderson, where Dr. Symmans helped develop the residual cancer burden system, for training.
Read More »Ins and outs of von Willebrand factor activity assays
January 2023—Guidelines for the diagnosis of von Willebrand disease, published in 2021, have raised questions about which von Willebrand factor activity assay laboratories should use.
Read More »For blood cultures, a lab’s new system and incubation time
January 2023—For the central microbiology laboratory serving Barnes-Jewish and four other hospitals in the St. Louis area, validating and implementing a new blood culture system and moving to a shorter incubation time came at a perfect time: right before the pandemic.
Read More »New starts: rapid-molecular pullback, fentanyl screen
January 2023—Respiratory viruses were up in most states when Compass Group members met online Dec. 6 with CAP TODAY publisher Bob McGonnagle, and some were looking to centralize their now decentralized rapid molecular testing. At least one system had already done so. In California, a new law requires fentanyl screening be included in drug screens in all general acute-care hospital lab settings.
Read More »Array of flow cytometry cases in new color atlas
January 2023—Due out this spring is the CAP’s Color Atlas of Flow Cytometry. It consists of 71 cases and provides examples of the full range of hematolymphoid diseases that can be productively analyzed by flow cytometric immunophenotyping. Its editors are David Dorfman, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital; William Karlon, MD, PhD, of the University of California San Francisco Medical Center; and Michael Linden, MD, PhD, of M Health Fairview-University of Minnesota Medical Center. CAP TODAY recently asked Dr. Dorfman a few questions about the atlas. His answers to our questions and a sample case follow.
Read More »Cytopathology in Focus: Adequacy in cytopathology: an overview with a focus on FNA of lymph nodes and mass lesions
January 2023—The definition of “adequate” per the Merriam-Webster dictionary is “sufficient for a specific need.” In cytopathology, it is defined by the quantity and quality of the cellular material sampled. The final interpretation of a cytopathology report is almost universally preceded by an adequacy statement. While the essence of “adequacy” stays the same, its application varies depending on the specimen type and the site sampled. Furthermore, in the current era of personalized medicine, the definition of adequacy has expanded from “enough cells to make a morphologic diagnosis” to “enough cells to make a diagnosis and perform ancillary studies.”
Read More »Cytopathology in Focus: Serous fluid cytopathology—recent progress and Yale’s experience
January 2023—In recent years, a standardized classification system for the cytology of serous body cavities has been proposed. The system, known as the International System for Reporting Serous Fluid Cytopathology (ISRSFC), published by Ashish Chandra, et al.,1 in 2020, aims to enhance the reproducibility of cytologic diagnoses, thereby facilitating clearer communication with clinicians. The diagnostic categories are as follows: I. Non-Diagnostic; II. Negative for Malignancy; III. Atypia of Undetermined Significance; IV. Suspicious for Malignancy; V. Malignant. Each progressive diagnostic category from I through V carries with it an increasing risk of malignancy. This brief review article aims to highlight the salient points of each diagnostic category and includes discussion of recent publications and our own institutional experience.
Read More »Volume? Space? Automation decisions in coagulation
January 2023—Automation and point-of-care, reflex, and viscoelastic testing were some of what came up when a group spoke with CAP TODAY publisher Bob McGonnagle in late November about hemostasis testing. Also tossed in: Results reporting to the EHR, which “can always be improved,” said Eric Salazar, MD, PhD, of University of Texas Health San Antonio. And D-dimer, one of the pandemic’s “health care heroes,” said Nichole Howard of Diagnostica Stago. Here’s what they said about all that and more.
Read More »The way forward for prehospital transfusion
December 2022—Ask Leonard Weiss, MD, what his favorite part of his schedule is, and he’s quick to answer that it’s the fieldwork: the helicopter and ambulance dispatches he accompanies once or twice a month as associate medical director of emergency medical services at the University of Pittsburgh Medical Center. Dr. Weiss, who is also assistant professor of emergency medicine and assistant medical director of Pittsburgh’s Stat Medevac service, says one of the UPMC emergency services he strongly supports is the prehospital transfusion of blood products. “Until recently, there wasn’t a lot of evidence to deploy its use on the ground as it is in the air, but thanks to extensive use by the military and scientific evidence of the value of prehospital transfusion,” he says, it is more likely to become part of some hospitals’ emergency medicine programs. The 911 ground-based transfusion program at UPMC and city of Pittsburgh EMS began in 2020. As Dr. Weiss and his UPMC colleagues acknowledge, however, myriad complexities come into play.
Read More »Urine test ordering—good and going for better
December 2022—Reflex urine culture algorithms have become common and have been shown to reduce urine culture utilization, but efforts to sharpen clinical decision support continue.
Read More »Digital pathology now, and where to from here
Nearly 800 registrants were at the Digital Pathology Association’s Pathology Visions meeting this fall, and 54 companies exhibited. “There was a great vibe at the meeting. People were mingling, collaborative. Digital pathology is picking up,” says DPA president Esther Abels. Her term as president will end this month and Liron Pantanowitz, MD, PhD, MHA, of the University of Michigan, will step in as president on Jan. 1.
Read More »In toxicology, unraveling the unexpected positives
December 2022—In toxicology testing, cross-reactive compounds, incomplete medical records, immunoassay performance, calibrator drift, and human error all play into unexpected positives.
Read More »New for waived-only labs: a custom GEN checklist
December 2022—Laboratories that provide only waived testing will now have a CAP accreditation program laboratory general (GEN) checklist tailored to waived test procedures, beginning with the 2022 checklist edition released in October.
Read More »Artificial intelligence in pathology: the tools, the talk
December 2022—In September, when CAP TODAY publisher Bob McGonnagle met with pathologists and representatives of companies to talk about laboratory information systems (“Lab information systems—where the needs are greatest,” https://bit.ly/LIS_112022), they talked also about artificial intelligence—innovations, clinical impact, how much interest there is. That part of their conversation follows.
Read More »Bright prognosis for brain injury biomarkers
November 2022—The lack of tools for assessing traumatic brain injury has long bedeviled physicians. There’s CT. And then? “This has been an unmet medical need for years,” says Ramon Diaz-Arrastia, MD, PhD, the John McCrea Dickson, MD, professor of neurology and director of the Clinical Traumatic Brain Injury Research Center, University of Pennsylvania Perelman School of Medicine. “As many of us know, it’s one of the major barriers that has hindered clinically advanced development of new therapies in TBI. And I think it’s pretty clear that the clinical evaluation alone leaves a lot to be desired.” “I am always frustrated that we have limited tools,” agrees Frederick Korley, MD, PhD, associate professor and associate chair for research in emergency medicine, University of Michigan Medical School, and scientific director, Massey TBI Grand Challenge, Weil Institute, University of Michigan. That’s now on the cusp of changing. Blood-based biomarkers for brain injury may not be bellying up to the bar just yet, but they are starting to raise the bar for how physicians assess TBI.
Read More »Is apolipoprotein B the best measure of CVD risk?
November 2022—The evidence in favor of measuring apolipoprotein B routinely, with other lipid parameters, is now so overwhelming, says cardiologist Allan Sniderman, MD, that he believes it’s unreasonable to deny patients the advantage of apoB. “If evidence is what counts,” he says, “then the care Americans receive should include apoB.”
Read More »Canadian pathology study finds high burnout prevalence
November 2022—Burnout among Canadian pathologists is prevalent, pain related for some, and workload driven for many. “There needs to be more of us,” says Julia Keith, MD, associate professor in the Department of Laboratory Medicine and Pathobiology at the University of Toronto.
Read More »In toxicology, puzzling out the unexpected negative
November 2022—In cases of unexpected negative results in toxicology testing, avoid overinterpretation, know your assays and providers, and don’t put off definitive testing when it’s needed, though it’s not a panacea.
Read More »The who, what, and when of respiratory virus testing
November 2022—In mid-October, flu was picking up, with high levels of activity in Texas, Georgia, the District of Columbia, South Carolina, Tennessee, and New York. Elsewhere, it was still on the lower side, with less known about what was to come but plans in place. And questions, too, about laboratory testing as it relates to SARS-CoV-2, “which is going to be a challenge,” says David Peaper, MD, PhD, D(ABMM), a member of the CAP Microbiology Committee.
Read More »No time to wait: How rapid NGS changed cancer care
November 2022—Rapid next-generation sequencing in a community hospital setting, performed by histotechnologists and interpreted by anatomic pathologists, is possible and paying off, and it “makes the pathologist a much more meaningful part of the precision oncology team,” says Brandon Sheffield, MD, of the Department of Laboratory Medicine, William Osler Health System, Brampton/Etobicoke, Ontario. “It has changed practice at our hospitals,” he says.
Read More »Lab information systems—where the needs are greatest
November 2022—What labs want and need from their lab information systems and what the missing pieces are in interoperability are what pathologists and LIS company reps talked to CAP TODAY publisher Bob McGonnagle about when they met online Sept. 12. “The biggest challenge is with device integration” in molecular testing, said J. Mark Tuthill, MD, of Henry Ford Health System. “We have million-dollar instruments and we’re still programming runs manually. We don’t have HL7 order feeds. We don’t have the ability to get result feeds outbound from those devices.”
The art and science of positive blood cultures
October 2022—It might be possible to tot up, using only the number of toes on an ordinary foot, how many labs are feeling full of vim and vigor these days, open to concepts like creative destruction and get those creative juices flowing and have fun with it—slogans once easily uttered but now tiring to enact. Nevertheless, Margie Morgan, PhD, D(ABMM), would like her colleagues to at least consider the possibility of inspiration in the microbiology laboratory. In particular, Dr. Morgan, medical director of microbiology and professor of pathology and laboratory medicine, Cedars-Sinai Medical Center, Los Angeles, has some thoughts about using a new automated system to facilitate rapid microbial identification from positive blood cultures. The Arc system, from Accelerate Diagnostics, is composed of the Arc module and blood culture kit and concentrates organisms recovered in positive blood cultures for direct testing on MALDI-TOF mass spectrometry. Dr. Morgan and colleagues have been using the system since February.
Read More »Platelet transfusions: safety, cost, and workflow
October 2022—The jury may no longer be out on whether pathogen reduction of platelet units reduces the risk of a septic transfusion reaction enough to replace culturing of platelet units.
Read More »Checklists now made to fit for next-gen sequencing labs
October 2022—As the diagnostic uses for next-generation sequencing have grown, so too has the length of the NGS section of the CAP molecular pathology accreditation program checklist. Now, with the release of the new checklist edition this month, NGS laboratories will find the NGS section in their customized checklists leaner, more relevant, and easier to read.
Read More »Sodium measurement—when the method matters
October 2022—William E. Winter, MD, D(ABCC), is blunt about whether to report a corrected sodium: He would worry if his name were on such a report. “I think you have to be careful about formulas,” he said in his “hot topic” talk at the AACC meeting in July.
Read More »Purchased for the pandemic? Rethinking instrumentation
October 2022—Who’s doing what with instruments purchased at the peak of the pandemic? That and next-generation sequencing are what CAP TODAY publisher Bob McGonnagle asked Compass Group members about when they met virtually on Sept. 6. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
A wait-and-watch season of respiratory viruses
October 2022—Influenza incidence and what it will mean for testing in this respiratory virus season is a wild card, as is how SARS-CoV-2 will evolve. In early September, SARS-CoV-2 prevalence was declining in parts of the United States. “And if you believe in the theory of viral interference,” says Michelle Tabb, PhD, chief scientific officer at DiaSorin Molecular, “it’s leaving the door wide open right now for something else to step in. We’ll see if that’s RSV, or flu A, or if it’s a new COVID variant.”
Read More »Scoring HER2 expression across the full spectrum
October 2022—HER2-low breast cancers are now of greater clinical interest, given Enhertu’s recent approval for use in treating such cancers. How to achieve accurate and reproducible results in scoring HER2-low tumors was at the center of a CAP TODAY webinar on new perspectives on the full spectrum of HER2 expression in breast cancer.
Read More »Enabling ‘the magic’ in hematology—eyes on what labs need
October 2022—New and better solutions for the hematology laboratory. That was at the center of a Sept. 2 virtual roundtable, led by CAP TODAY publisher Bob McGonnagle. With him were Jonathan Galeotti, MD, of the University of North Carolina School of Medicine, and representatives of Sysmex America, Siemens Healthineers, Beckman Coulter, and CellaVision. “It’s a new era in terms of what can happen in hematological data,” said Fernando Chaves, MD, global head of hematology, Siemens Healthineers.
Read More »Highs, lows of tumor mutation burden testing
September 2022—It may not be the oldest story in the world, but in clinical laboratories it’s an oft-told tale: Tumor meets biomarker; drug meets companion diagnostic; both meet FDA approval; clinicians meet with patients offering new hope—and those in the lab are left trying to figure out how to make it all work. That story is playing out again in the realm of measuring tumor mutational burden. In mid-2020 the FDA approved pembrolizumab as a new treatment option in adult and pediatric patients with TMB-high (≥10 mutations/megabase) solid tumors, as determined by the FDA-approved FoundationOne CDx assay. “That doesn’t sound too controversial, right?” says Alain Borczuk, MD, vice chair of anatomic pathology and director of oncologic pathology, Northwell Health Cancer Institute. “It’s not the only way in, but it’s one of the ways in. If you’re arguing for your patient that this is the biomarker that makes them eligible for the drug, then the next questions will be, What was the number? And what was the test?” And it’s off to the races.
Read More »As blood supply tightens, so too does mitigation
September 2022—Picture a performer juggling tenpins while walking a high wire, knowing that a hurricane looms. Add a safety net that could disappear at any time. That’s a sense of what hospital transfusion services experience in maintaining enough blood products to meet patients’ needs.
Read More »BNP and NT-proBNP: how they differ, what it means
September 2022—Which biomarker for heart failure should be used—BNP or NT-proBNP—and does it matter? That’s the question Christopher W. Farnsworth, PhD, set out to answer in his “hot topic” talk (one of a trio) at the AACC meeting in July.
Read More »New data on rapid rule-out using high-sensitivity cTnT
September 2022—A single high-sensitivity cardiac troponin T measurement below the limit of quantitation of 6 ng/L is a safe and rapid method to identify a substantial number of patients at low risk for acute myocardial injury and infarction, say the authors of a recently published study.
Read More »What’s required in ’23 for predictive marker tests
September 2022—Beginning next year, two additional predictive marker tests will require enrollment in proficiency testing (PT), but for any predictive marker using immunohistochemistry or in situ hybridization, only laboratories that perform both staining and interpretation must participate in CAP-accepted PT.
Read More »U.S. blood supply steadier but still short
August 2022—Blood is a precious resource and shouldn’t be treated as a commodity. That’s the consensus in the blood banking community, in line with a longstanding conviction that volunteer donations should remain at the blood system’s core. But as the worst of the pandemic appears to have passed, discussion of blood shortages has increasingly drawn on the vocabulary of commerce, and the warnings about the blood supply have been rife with references to supply chain problems that go beyond the need for more donations. Crises in the blood supply are nothing new, and while the health care system strives to stay prepared, the pandemic threw novel commercial and logistical factors into the mix, in some ways jumbling the expected order of a crisis for blood services. Hospitals scrambled to cope with a surge of COVID-19 patients while the spread of infection caused thousands of blood drives to be canceled, so there was a steep drop in supply of blood products, says Pampee Young, MD, PhD, chief medical officer, biomedical services, American Red Cross.
Read More »Autoverification: lessons drawn from a core lab
August 2022—Just as there is scant room in this world for pink “While You Were Out” notepads, paper checks, or the copying skills of Bartleby the Scrivener, laboratories would do well to leave manual result verification in the past.
Read More »A practical approach to borderline melanocytic neoplasms
August 2022—In cases of borderline melanocytic neoplasms, which have overlapping histopathologic features of benign and melanocytic lesions, additional immunohistochemical studies sometimes help to differentiate the two. But a subset of lesions will show overlapping features.
Read More »Looking for lab staff here, there, and overseas
August 2022—Higher wages help to fill open positions, when they can be offered, but in a labor market that’s as tight as ever, they’re often just a start. That’s why many laboratories are casting wider nets, though the hiring solutions tend to be long term.
Read More »Infectious diseases of the gut
August 2022—The atypia in Epstein-Barr virus-positive mucocutaneous ulcers can mimic diffuse large B-cell lymphoma or classical Hodgkin lymphoma, a diagnostic pitfall that can result in overtreatment. And esophageal ulcers in immunocompromised patients should trigger cytomegalovirus immunohistochemistry in addition to GMS and herpes simplex virus-1 and -2 stains.
Close-up on HER2 alterations in advanced NSCLC
August 2022—HER2 is a known oncogenic driver and emerging biomarker in non-small cell lung cancer, and while the therapeutic implication is not yet fully known in NSCLC, “we need to pay attention to it,” said Fred R. Hirsch, MD, PhD, executive director of the Mount Sinai Center for Thoracic Oncology and associate director, Tisch Cancer Institute, in a CAP TODAY webinar sponsored by Daiichi-Sankyo and AstraZeneca.
Read More »Savings, schedules, new automation—labs weighing it all
August 2022—Running into the reality of staffing. Those are the words of a pathologist who said in the most recent Compass Group roundtable that its health system is making a push to obtain and test specimens “as close to home as possible.” Another Compass Group member said planning is underway for the new hospitals his system is going to build, “but we don’t know where staff will come from.” Here is what they and others told CAP TODAY publisher Bob McGonnagle on July 5.
Read More »Cytopathology in focus: Protocol for reporting cervicovaginal cytology specimens
August 2022—The protocol for the reporting of cervicovaginal cytology, the first in a series of CAP cytopathology protocols, became available for use in a synoptic format on June 22. This protocol is a collaborative effort, based on input from past and present members of the CAP Cytopathology Committee and prepared in conjunction with the CAP Pathology Electronic Reporting Committee. It was presented via webinar to the CAP House of Delegates on March 31. A two-week open comment period followed; all comments were reviewed and appropriate changes were incorporated into the protocol.
Read More »Cytopathology in focus: Advances in detection of mesothelioma in cytology pleural fluid specimens
August 2022—The ability to make a definitive diagnosis of mesothelioma on pleural fluid cytology has been questioned and debated for a long time. The 2018 American Society of Clinical Oncology clinical guidelines limit the cytological diagnosis of pleural fluid specimens only as an initial screening test for mesothelioma. Monaco, et al., discuss in their article the appropriate use of ancillary studies (immunohistochemistry and fluorescence in situ hybridization studies) to make a definitive diagnosis of mesothelioma in small tissue samples, which are often processed as cell blocks. The authors recommend a stepwise approach starting with immunohistochemistry for BAP1 and, next, MTAP in cases of atypical mesothelial proliferations where the suspicion for malignant mesothelioma is high.
Read More »Transgender care, in and beyond the lab
July 2022—Gabrielle Winston-McPherson, PhD, could be talking about almost any aspect of laboratory medicine as she recounts how the Henry Ford Health chemistry division, in which she is associate director, has identified a patient care need. She talks about the desire to improve health outcomes. Identifying problems in the preanalytical process. Appropriate test utilization. Putting together a team to develop training material. Assembling data and information prior to implementation. Informatics challenges. And, naturally, the perpetual financial concern of ensuring allocation of limited resources. How else would she—or any other laboratory professional—talk about the lab’s role in transgender health care? In fact, there are many other ways to discuss the topic. “It’s been in the news a lot these days, obviously,” says Matthew Krasowski, MD, PhD, clinical professor and vice chair, clinical pathology and laboratory services, University of Iowa Hospital and Clinics. In fact, there are many other ways to discuss the topic.
Read More »Monoclonal gammopathies: which tests and why
July 2022—Variability in testing for monoclonal immunoglobulin proteins was in part what led to the new guideline on laboratory detection and initial diagnosis of monoclonal gammopathies, published in May.
Read More »Histology lab tips for top-tier whole slide images
July 2022—Good slides in, good images out. Liron Pantanowitz, MD, MHA, explained how to get those good images in a recent webinar on preanalytics quality control in digital pathology, sponsored by Sunquest and made available by the Association for Pathology Informatics.
Read More »Emergency department tests HIV screening strategy
July 2022—Thanks to more than two years’ experience with SARS-CoV-2, perhaps at no point in U.S. history has the general public been as aware of antigen and PCR testing, and the difference between them, as it is now.
Read More »‘A struggle every day’—outpatient center decisions
July 2022—A time of tough choices. A complex dance. This is how Compass Group members on a call with their colleagues, led by CAP TODAY publisher Bob McGonnagle, describe what it’s like to cover outpatient centers amid severe staff shortages. “We are consuming significant resources to get all our locations staffed,” one member says. Another predicts: “We will not be out of this staffing situation for 10 years.” Here is more of what they and others talked about on June 7 as COVID positivity rates were up and monkeypox was in the news. The Compass Group is an organization of not-for-profit IDN system laboratory leaders who collaborate to identify and share best practices and strategies.
Read More »One hospital’s story: Ins and outs of low titer O whole blood use in trauma
July 2022—Myriad questions had to be answered and plans made to put low titer O whole blood in the trauma bay at Thomas Jefferson University Hospital. Julie Katz Karp, MD, associate professor and director of transfusion medicine, reported why, when, and how it was done and where they stand today, in a process she describes as “a never-ending series of hoops.”
Read More »What’s bugging the gut? A team approach
July 2022—Gut pathogens, their histologic features, and a GI pathology and microbiology team approach to diagnosis were the focus of a CAP21 session, “What’s Bugging the Gut?” Maryam Zenali, MD, Alina Iuga, MD, and Christina Wojewoda, MD, presented a series of cases and highlighted the features, the differential diagnoses, and the integrated workups. Three of their cases follow here, with others to be reported in an upcoming issue.
Read More »How close to patients? Cost, quality, competition
July 2022—Point-of-care versus centralized testing, and automation, IT, and staffing. It all came together as industry executives and a laboratory director and a former medical director met with CAP TODAY publisher Bob McGonnagle on May 25, as CAP TODAY’s list of chemistry and immunoassay analyzers was going together. “I don’t worry much about the machines or reagents,” thanks to good-quality practices, said André Valcour, PhD, MBA, DABCC, of Labcorp, who noted the real focus is quality of information and information transfer. Susan Fuhrman, MD, formerly of OhioHealth, said, “We should always give our clinicians as much information as we can accurately produce and our reports should be as clear as we can make them.” And of the staffing crisis: “We have a perfect storm,” she said. Here is more of what they and the others had to say.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management