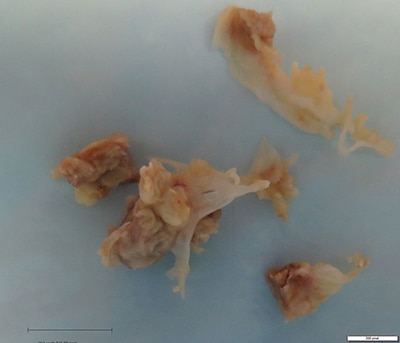
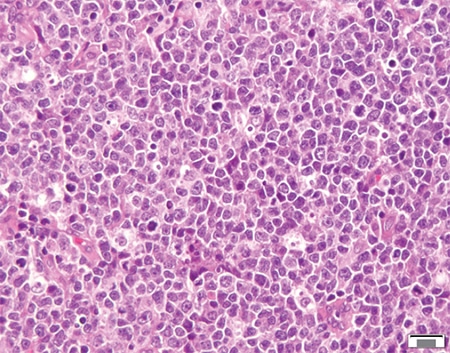
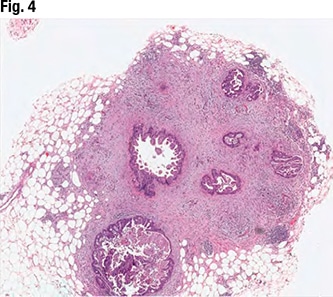
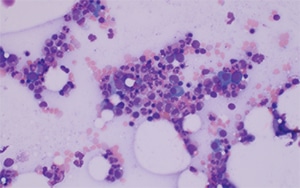
April 2021—A 71-year-old female with a history of asthma and hypertension initially presented to her local hospital complaining of shortness of breath. She was found to be pancytopenic with severe anemia (hemoglobin 5 g/dL). She was subsequently transferred to a tertiary care facility for further evaluation. Bone marrow biopsy revealed a hypercellular marrow composed of 72 percent blasts. Flow cytometric analysis revealed a B-lymphoblast immunophenotype with expression of CD34, dim CD45, CD19, CD79a, CD22, HLA-DR, TDT, CD200, CD33, and dim CD13.
Read More »2020 Issues
In SARS-CoV-2, small steps but big wins
December 2020—By its very nature, the global pandemic has forced laboratories to look far and wide, to bring binoculars, in essence, to their views of supply chains, testing platforms, personnel, and the like. As COVID-19 churns on, some labs are looking through a tinier lens as well. These labs aren’t trading their binoculars for a jeweler’s loupe, exactly, but they have found small and significant success stories closer to home. Like so many others, Erin Graf, PhD, D(ABMM), has confronted a spinning roulette wheel since the pandemic’s start. In a talk she gave in an AMP webinar in October, Dr. Graf posted a vibrantly colored wheel titled, “Which supply chain issue will impact us this week?” Each segment contained a phrase familiar to everyone in 2020, ranging from “swabs” and “sheep blood agar” to “pipette tips” and “chlamydia and gonorrhea tests.” As she surveys these continuous claims on her attention, Dr. Graf says, “I think none of us could have ever thought that COVID would have an impact on all these arms of the testing that we do.”
Read More »Digging into drug’s effect on aPTT-based assays
December 2020—As ongoing studies reveal the merits of emicizumab for hemophilia A patients—fewer bleeding episodes, longer duration between treatments—laboratories need to be alert to the drug’s effect on coagulation testing.
Read More »How to right the wrongs of clinical decision support alerts
December 2020—Always think about timing, maintain a log of malfunctions, and make the right decision the easy decision. These are a few of the clinical decision support tips that Ronald Jackups Jr., MD, PhD, and Amanda Blouin, MD, PhD, presented last month in a CAP20 session.
Read More »Journeys to alternative SARS-CoV-2 strategies
December 2020—In Colorado, Joan Coleman, MBA, MT(ASCP), and her UCHealth colleagues launched an expansive pooled testing program this summer that, after much work, worked well—until it didn’t, thanks to rising positivity rates in October.
Read More »Identifying respiratory pathogens: Pneumonia panel studied against standard of care
December 2020—In an evaluation performed at Washington University in St. Louis and published recently, BioFire’s FilmArray pneumonia panel was found to have strong agreement with standard-of-care methods in identifying viral and bacterial targets in 200 lower respiratory tract specimens (Webber DM, et al. J Clin Microbiol. 2020;58[7]:e00343–20). It was also found to have strong agreement with the BioFire upper respiratory panel for common targets, making it unnecessary to perform both. In comparison to standard-of-care methods, it has the potential to detect more Staphylococcus aureus and Haemophilus influenzae and to detect more antimicrobial resistance, particularly at low organism concentrations or in mixed cultures.
Read More »AMP case report: A vanishing twin as an explanation for discordant fetal sex results with NIPS and ultrasound
December 2020—Circulating cell-free DNA in the blood of pregnant women is derived from both maternal tissues and the placenta.1 As a result, cfDNA isolated from maternal plasma can be used for noninvasive prenatal screening (NIPS) to identify fetal autosomal aneuploidies (trisomies 13, 18, and 21) and sex chromosome aneuploidies (SCAs). For fetal autosomal aneuploidies, NIPS offers higher detection rates and lower false-positive rates than traditional screening methods, such as maternal serum screening and nuchal translucency.2 NIPS is the only screening option available for SCAs, such as Turner syndrome (45,X) and Klinefelter syndrome (47,XXY), which do not present with ambiguous genitalia on fetal ultrasound.3
Read More »Solving problems, restricting orders: Compass on COVID
December 2020—The Compass Group reconvenes to share the latest on SARS-CoV-2 testing—this time on Oct. 6 and again by Zoom. What they said about supplies, labor, and flu follows. Serology testing too: “It’s the one test we have loads of and the one test they don’t use a lot of,” said Heather Dawson of Allina Health in Minneapolis. CAP TODAY publisher Bob McGonnagle led the roundtable. With Dawson were Walter Henricks, MD, of Cleveland Clinic; Jennifer Laudadio, MD, of the University of Arkansas for Medical Sciences; Joseph Baker of Baylor Scott & White; Judy Lyzak, MD, MBA, of Alverno; Susan Fuhrman, MD, of OhioHealth; Dan Ingemansen and Rochelle Odenbrett, MT(ASCP), MBA, of Sanford Health; Janet Durham, MD, of ACL Laboratories; Diana Kremitske, MS, MHA, MT(ASCP), of Geisinger; Darlene Cloutier, MSM, MT(ASCP), HP, of Baystate; Stan Schofield of NorDx; Clark Day of Indiana University Health; Tylis Chang, MD, of Northwell; and John Waugh, MS, MT(ASCP), of Henry Ford. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies.
Read More »Urinalysis: ‘a field with the potential to do more’: pathologist, two companies talk about urinalysis now and what’s needed
December 2020—What could improve urinalysis operations in your laboratory? That’s a question CAP TODAY publisher Bob McGonnagle asked Megan Nakashima, MD, of the Cleveland Clinic when she talked in October with him and two others: Carl Trippiedi of Sysmex and Matt Rhyner, PhD, MBA, of Beckman Coulter. Their conversation took place as CAP TODAY’s 2020 product guide to urinalysis instrumentation was taking shape. What they had to say follows.
Read More »From the President’s Desk: The year that wouldn’t end
December 2020—As we reach the end of December, I am taking a look back at this year and wondering: How long was 2020 anyway? My calendar says 12 months, but if you’re like me, 2020 seems like it has already had about 24 in it and I’m still counting. I’ve even taken to using “2020” as an expletive. When I think of the key themes of the past year, most are quite negative. The pandemic, virtual meetings, and extremely challenging legislation just to name a few. In the spirit of hoping the worst is behind us—and to remind ourselves how much we worked to make the best of a bad situation—let’s take a quick tour of the major difficulties we faced in 2020.
Read More »Clinical pathology selected abstracts
December 2020—The National Academy of Medicine estimated that approximately 30 percent of U.S. health care spending constitutes nonvalue-added waste. This waste may be generated through unnecessary laboratory tests and services, inefficiency of care delivery, excessive administrative costs, and high prices. A goal of medical educators is to inform undergraduate medical students about health care management and health care delivery to make them better stewards of cost-effective, high-value care (HVC). The authors described the results of a needs analysis to inform the design of an online case-based educational tool for teaching laboratory stewardship to medical students. To this end, they conducted a needs assessment that included semi-structured interviews of core clerkship directors and residency program directors, a national survey of the Undergraduate Medical Educators Section of the Association of Pathology Chairs, and a review of existing online resources for teaching HVC. Their results showed that all of the core clerkship directors and residency program directors thought that teaching laboratory stewardship as part of the undergraduate medical education (UME) curriculum was important. The two major themes that emerged from the analysis to enhance laboratory stewardship education were appropriate test ordering and interpretation. The authors also found several organizations that provide HVC education through online modules or clinical cases.
Read More »Anatomic pathology selected abstracts
December 2020—It can be difficult to distinguish metastatic melanoma from melanocytic nevi in lymph nodes. Because diffuse IHC PRAME (preferentially expressed antigen in melanoma) expression is detected in the majority of primary and metastatic melanomas, but rarely in nevi, the authors conducted a study in which they hypothesized that PRAME could be a useful adjunct marker for the diagnosis of melanocytes in lymph nodes. They examined 45 nodal melanocytic deposits comprising 30 nodal nevi and 15 melanoma metastases. The latter were not straightforward from a diagnostic perspective because they coexisted with nodal nevi or were present in perinodal fibrous tissue. All nodal nevi were negative for PRAME and all melanoma metastases were diffusely positive for PRAME IHC.
Read More »Molecular pathology selected abstracts
December 2020—Next-generation sequencing-based mutation testing of various cancer types is clinically indicated and widely used to diagnose disease, inform potential therapeutic targets, prognosticate disease course, and monitor responses to targeted and nontargeted therapies. The genetic variants discovered by tumor-based next-generation sequencing (NGS) can be somatically acquired by the neoplastic cells or a fixed inherited component of the patient’s germline genome. Distinguishing the germline versus somatic status of tumor NGS-defined variants is of significant clinical importance not only for patient care but possibly for patients’ families. Because many cancers have a substantial inherited component, the discovery of a pathogenic germline mutation by tumor-based NGS may have substantial familial implications. For example, being aware of a cancer risk allele, such as BRCA1, can lead to the use of highly effective interventions to prevent or treat the related cancer in family members. Consensus guidelines recommend germline genetic testing only for those cancer patients who have a clinical presentation or family history suggestive of hereditary disease.
Read More »Q&A column
Q. Can a heel stick for a basic metabolic panel with magnesium and phosphorus be performed on a two-month-old baby? Read answer. Q. Due to nationwide supply shortages affecting COVID-19 and other testing in the laboratory, we are concerned about using up critical supplies when assessing competency. Do you have suggestions or strategies we can use? Read answer. Q. When a patient is admitted to our hospital, we collect MRSA nares PCR, MRSA axilla by culture, MRSA groin by culture, and vancomycin-resistant Enterococcus by PCR for infection control purposes. Many surrounding facilities have told us they have removed the axilla and groin cultures, but no references were cited to support removing these procedures. Our facility would like to follow the practices of other hospitals, but our providers would like a reference to cite. Are there best practices or benchmarks from an infection control and microbiology point of view that would allow us to remove the axilla and groin MRSA screen cultures? Read answer.
Read More »Newsbytes
New NovoPath CEO settled in and taking questions December 2020—CAP TODAY publisher Bob McGonnagle recently spoke with Promise Okeke, who took the helm as CEO of NovoPath last summer. Here’s what Okeke had to say about NovoPath’s case distribution module, customer service, and the advantages of offering a best-of-breed system, among other topics.
Read More »Put It on the Board
December 2020—Antimicrobial Susceptibility Testing: Monitoring and Trend Analysis is a new CAP program that is beginning to roll out to laboratories this month. The CDC guidance for antibiotic stewardship consists of seven core elements to address resistance-associated risks, one of which points to the importance of laboratory collaboration, communication, and AST reporting practices to the success of stewardship programs. According to this core element, the laboratory must provide information to guide discussions on the potential implementation of test interpretive criteria, such as changes in antibiotic breakpoints, that might affect antibiotic use.
Read More »Making peace with saliva, pooled testing
November 2020—Adam Barker, PhD, D(ABMM), was ready to call it quits. For weeks, he had been working to bring saliva-based SARS-CoV-2 testing to ARUP Laboratories and the University of Utah. Dr. Barker, director of ARUP’s COVID-19 rapid response lab, and his colleagues had done studies comparing saliva with nasopharyngeal swabs, which seemed to be following the flight of the passenger pigeon out of existence. They had wrestled with the FDA over emergency use authorization. They’d developed their own transport media, since that supply was also becoming extinct. He had begun building kits for saliva collection and figured out what sample size worked best. Kits had been delivered to collection sites on campus, and staff were being trained in their use. He was, in other words, creating a laboratory success story, one of the many that have been written since March. He was not basking in this fact. “I have to tell you: I lost so much sleep because of saliva,” says Dr. Barker, who is also director, ARUP Institute for Clinical and Experimental Pathology.
Read More »Checklist, CLIA line up on COVID reporting
November 2020—It’s been well understood since the Ten Commandments that rules that appear simple in theory can be fiendishly complex or even impossible to execute. The pandemic is providing a perfect example of that in the laboratory world, but with added twists, at least for now.
Read More »Three at AACC: rapid STI testing, toxicology, biosafety
November 2020—Point-of-care testing for sexually transmitted infections, toxicology investigation, and biosafety practices are three of the hundreds of topics that will come online next month during AACC’s virtual annual meeting.
Read More »Fewer urine cultures — series of changes add up
November 2020—Five years after putting in place a urine reflex algorithm at Barnes-Jewish Hospital in St. Louis, and many tweaks later, Melanie Yarbrough, PhD, D(ABMM), D(ABCC), has tips to share on how to increase the odds for success in reducing the number of urine cultures.
Read More »At POC and in lab, 2 new checks on SARS-CoV-2 testing
November 2020—The CAP released in September its proficiency testing program for SARS-CoV-2 antigen testing, with the first shipment to laboratories set for Nov. 30. It also introduced recently a Quality Cross Check program that makes it possible for labs performing nucleic acid amplification testing for SARS-CoV-2 to monitor performance across multiple instruments, in compliance with the CMS directive prohibiting proficiency testing on multiple instruments.
Read More »Compass on COVID: What test for whom and when—lab leaders talk
November 2020—Testing saliva, stocking up, and expanding capacity were top of mind when members of the Compass Group convened by Zoom on Sept. 1 for a second COVID-19-related call with CAP TODAY publisher Bob McGonnagle. Antigen testing, too, came up, and the question to answer there, said Susan Fuhrman, MD, of OhioHealth, is why the test is performed and what will be done with the result. That and more—testing for patients undergoing treatment for cancer, flu season—were up for discussion. Others on the call were Greg Sossaman, MD, of Ochsner; Lauren Anthony, MD, and Heather Dawson of Allina; Sarah Province and Julie Hess of AdventHealth; James Crawford, MD, PhD, of Northwell; Stan Schofield and Robert Carlson, MD, of MaineHealth; Sterling Bennett, MD, MS, of Intermountain; John Carey, MD, of Henry Ford; and Pamela Murphy, PhD, APRN, of MUSC Health. The Compass Group is an organization of not-for-profit IDN system lab leaders who collaborate to identify and share best practices and strategies. (For our coverage of their first call with CAP TODAY, see “Compass points chart the pandemic,” September 2020.) Here is what they told us on Sept. 1.
Read More »IT in a pandemic year, now and what’s ahead: interfaces, analytics, telepathology—seven weigh in
November 2020—Information technology from a COVID-19 perspective. What has been the impact on IT, and what change is yet to come? That is what seven people who met virtually on Sept. 10 talked about with CAP TODAY publisher Bob McGonnagle. They are James Harrison, MD, PhD, of the University of Virginia; J. Mark Tuthill, MD, of Henry Ford; Stephen Hewitt, MD, PhD, of the National Cancer Institute; Bob Dowd of NovoPath; Michelle Del Guercio of Sunquest; Curt Johnson of Orchard; and Brian Gunderson of Roche. You will see here, in the conversation that follows, where their focus is as the crisis continues.
Read More »AMP case report: Role of lymphoma sequencing panel in diagnosis of pediatric-type follicular lymphoma
November 2020—Pediatric-type follicular lymphoma (PTFL) is a rare form of lymphoma that was recognized as a new diagnostic entity in the revised 2016 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. The classic features of PTFL include male predominance, localized stage I lymphadenopathy, blastoid morphology, high proliferation index, and exceedingly good response rate to local excision.
Read More »From the President’s Desk: Gratitude all around
November 2020—Despite the circumstances that forced us to shift to a virtual event for our annual meeting this year—and my sincere hope we will never have to do so again—I couldn’t be more proud of how everyone in the CAP worked hard to adapt to the COVID-19 pandemic and ensure the best experience possible for all of our pathologists. As I write this, the annual meeting has just concluded. While every conference we hold requires tremendous effort, this was a different event, a different horse race. The work and persistence that went into making our virtual meeting a success were extraordinary.
Read More »Clinical pathology selected abstracts
November 2020—Smoking is a leading cause of death in the United States and is associated with many postoperative complications, including increased transfusion needs. Toxins in tobacco that create free radicals that damage the arterial walls and make them more susceptible to rupture and bleeding may be the link between smoking and surgical bleeding. Smoking also impairs tissue healing after surgery, most likely due to reduced oxygenation and altered function of inflammatory cells during the healing process. This may impact bleeding risk in the immediate postoperative period. The authors conducted a study in which they queried the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) Participant Use Data File 2007–2016, which contained data from up to 680 U.S. hospitals, to test the hypothesis that smoking is associated with a higher risk of bleeding in various surgical procedures.
Read More »Molecular pathology selected abstracts
November 2020—Sporadic vascular malformations are congenital malformations of arteries, veins, capillaries, or lymphatic vessels, or a combination of these, and are associated with significant morbidity. The majority of them are caused by postzygotic somatic pathogenic variants in oncogenes in the PI3K-MTOR and RAS-MAPK pathways, including within PIK3CA, TEK, MAP2K1, BRAF, and KRAS. Investigators have assessed whether therapeutic agents targeting these pathways should be used to augment or replace traditional surgical management. But because these somatic variants are restricted to cells within the tissue of vascular malformations (VM), it is necessary to conduct genetic testing on the surgically resected tissue to qualify patients for trials of targeted therapies. Approximately 10 percent of cell-free DNA (cfDNA) originates from endothelial cells.
Read More »Anatomic pathology selected abstracts
November 2020—Neuroendocrine neoplasms range from well to poorly differentiated and indolent to highly aggressive. The site of origin in metastatic neuroendocrine neoplasms has therapeutic and prognostic implications. SATB2 is a transcriptional regulator involved in osteoblastic and neuronal differentiation and a sensitive and specific marker of colorectal epithelium. The authors conducted a study to evaluate the expression of SATB2 in neuroendocrine neoplasms from various primary sites and its utility as a marker for determining the site of origin of these neoplasms. SATB2 IHC was performed on 266 such neoplasms, including lung small cell carcinomas (n = 39) and carcinoids (n = 30), bladder (n = 21) and prostate (n = 31) small cell carcinomas, and gastrointestinal/pancreatic neuroendocrine neoplasms of various primary sites (n =145) consisting of well-differentiated neuroendocrine tumors (WDNET, n =124) and poorly differentiated neuroendocrine carcinomas (PDNEC, n = 21).
Read More »Pathology informatics selected abstracts
November 2020—Telepathology is a leading application for digital pathology. The ability to easily share a digital image in practice offers pathology laboratories clinical, operational, and financial benefits. This is best demonstrated by the longstanding success of telepathology in allowing pathologists to remotely perform intraoperative consultations—that is, to read frozen sections. Neuropathologists were one of the first specialists to leverage digital pathology for this clinical use. The authors, all of whom were from the University of Pittsburgh Medical Center, performed teleneuropathology at their institution, which implemented the practice 17 years ago.
Read More »Q&A column
Q. What can laboratories expect to see after a medication such as Narcan is given for an opioid overdose? Read answer. Q. There are conflicting views among my colleagues regarding the meaning of initial competency assessment. Some think that using a training checklist for new staff counts as the initial competency assessment because we are signing off that staff are competent to perform patient testing and report results. Others believe an initial competency assessment is done shortly after training is completed, followed by the mid-cycle/six-month competency assessment and annual competency assessment. Please clarify. Read answer. Q. How do you calculate RDW-SD and RDW-CV values in dimorphic anemia cases on the Sysmex XN-3000? Most of the dimorphic anemia cases report a masked parameter. Read answer.
Read More »Newsbytes
November 2020—As LigoLab was designing a direct-to-consumer portal for laboratory testing early this year, company CEO Suren Avunjian turned his focus to when in 2021 he would release it, not knowing what was around the corner. But as the number of COVID-19 cases grew to pandemic proportions, Avunjian realized he could and should redesign the portal to streamline SARS-CoV-2 testing.
Read More »Put It on the Board
November 2020—In an Association for Molecular Pathology survey, 62 percent of U.S. labs reported using only commercial testing kits with FDA EUA for SARS-CoV-2 molecular testing. Five percent reported using laboratory-developed tests only, 26 percent said they were using a combination of LDTs and EUA commercial kits, and six percent reported using LDTs, IRB-approved/non-EUA assays, and commercial kits. Less than one percent reported using a combination of LDTs and IRB-approved/non-EUA assays or a combination of IRB-approved/non-EUA assays and commercial kits.
Read More »Flu mounts COVID’s bustling stage
October 2020—Barely a half year into the pandemic’s presence in the United States, history has already begun pressing down on SARS-CoV-2 testing. Like an actor playing Hamlet, it’s been difficult not to feel the burden of past performances when preparing for the months ahead. Now, at the start of fall, that also means readying for the return of influenza. Here, even longer experience has shown that each new season is, indeed, a new season. As in the theater world itself these days, planning for what lies ahead feels tempest-tossed. Plans are being laid. Discussions continue. Creativity abounds, and hard work persists. The season shall unfold. But no one knows how it will look until the curtain—or whatever is passing for one this year—goes up. Poor Hamlet is troubled enough to fill the stage for hours—it is, in fact, Shakespeare’s longest play. Yet he’s just one man. Laboratories this fall are absorbing the slings and arrows of two roles simultaneously. Can they prepare for both parts (think Richard II and III sparring on the same stage) with confidence?
Read More »To fast or not to fast? Fat is the question
October 2020—For nearly five decades, clinicians and laboratories aiming to screen LDL cholesterol (LDL-C) in adults to assess cardiovascular disease risk have contended with a problem generally beyond their control: lack of assurance that patients told to fast before a blood specimen is collected for lipid testing have indeed fasted.
Read More »LSU autopsy findings point to endothelium as target in heart
October 2020—Autopsies conducted at University Medical Center in New Orleans on 22 patients who died from SARS-CoV-2 infection found not the expected typical inflammation of the heart muscle associated with myocarditis but instead scattered individual myocyte necrosis.
Read More »Higher CVD risk, or lower risk? hs-cTn in diabetes
October 2020—When Elizabeth Selvin, PhD, MPH, of Johns Hopkins Bloomberg School of Public Health, began her studies of high-sensitivity cardiac troponin assays, they had not yet been approved in the U.S., as they are now, for use in diagnosing myocardial infarction. But some of her studies and those of Amy K. Saenger, PhD, DABCC, medical director of clinical laboratories and director of clinical chemistry at Hennepin County Medical Center in Minneapolis, take high-sensitivity cardiac troponin in a new direction by exploring its potential use as an aid in monitoring cardiovascular risk in the general population.
Read More »Uncharted season forges new paths for all hands
October 2020—Planning for respiratory season is always tricky but never more so than this year. “Uncharted territory for influenza” is how Frederick Nolte, PhD, D(ABMM), of the Medical University of South Carolina, describes the prospect of testing for influenza at the scale labs have been testing for SARS-CoV-2.
Read More »Molecular methods shown to push cases forward: Case studies in hematopathology
October 2020—B-ALL with aberrant expression of myeloid markers should be investigated further for specific gene abnormalities, including ZNF384 rearrangements, and microarray analysis may play an important role.
Read More »AMP case report: TET2TET— reconciling conflicting genomic reports
October 2020—After 20 years of CAP advocacy, synoptic reporting in surgical pathology is ubiquitous. This came about in part by fiat and in part by all parties agreeing on the importance of standardization for patient care. The merits of some elements remain controversial. Molecular pathology, a newer discipline, does not offer the scope for creative writing once available in surgical pathology.
Read More »MGMT promoter methylation: assays, implications
October 2020—With MGMT gene promoter methylation observed in about 50 percent of glioblastomas, it remains a biomarker of strong clinical interest in routine practice, even though it’s not the sole determinant in decisions related to therapy. PCR and pyrosequencing are the most commonly used assays, and there’s a technique that is not yet mainstream but gaining interest, said Tejus A. Bale, MD, PhD, assistant attending pathologist in the Department of Neuropathology and Diagnostic Molecular Pathology, Memorial Sloan Kettering Cancer Center. Dr. Bale spoke June 30 in the first of a series of Association for Molecular Pathology webinars on emerging and evolving biomarkers.
Read More »From the President’s Desk: An access to care issue
October 2020—Many CAP TODAY readers know about my fondness for horse racing, so it will come as no surprise that I thoroughly enjoyed the belated running of the Kentucky Derby last month. I even made a few dollars from a small bet, which may be the only positive financial news I get in a year marked by COVID-19 and the threat of impending reimbursement cuts.
Read More »Clinical pathology selected abstracts
October 2020—Early in the COVID-19 pandemic, some reports linked ABO blood type to severity of the disease and test positivity. Among these were reports that blood type A was associated with a higher risk for SARS-CoV-2 infection and blood group O with a lower risk of infection and mortality. However, there is a paucity of data regarding the relationship between ABO blood type and severity of COVID-19. Therefore, the authors conducted a large multi-institutional observational study to determine if there is an association between ABO blood type and severity of COVID-19 and if those with specific blood types are more likely to test positive for the disease. For the study, they used a large multi-institutional database of adult patients who tested positive for SARS-CoV-2 at five major hospitals in Massachusetts from March 6 to April 16. The authors evaluated hospitalization, intubation, and death for an association with blood type.
Read More »Anatomic pathology selected abstracts
October 2020—Immune checkpoint inhibitors are frequently used to treat a variety of solid tumors. These drugs involve upregulation of cytotoxic T cells, which can lead to immune-related adverse events, including those involving the gastrointestinal tract. The authors conducted a study to characterize the histological features of immune checkpoint inhibitor therapy-associated gastritis. Gastric biopsies from patients on immune checkpoint inhibitor therapy who had clinical suspicion of drug-associated gastrointestinal injury were identified. The predominant histological pattern of injury, distribution of injury, degree of tissue eosinophilia, and prominence of apoptosis were recorded.
Read More »Molecular pathology selected abstracts
October 2020—An increase in the number of copies of a gene, or amplification, is regarded as the most common gain-of-function alteration across various cancer types. The authors developed a bioinformatics tool (Amplicon Architect) to identify extrachromosomal oncogene (ecDNA) amplification from whole genome sequencing (WGS) data based on three characteristic features: circularity of ecDNA, absence of a centromere, and high levels of amplification. The tool was validated in 44 cancer-derived cell lines known to have ecDNA. A combination of centromeric and noncentromeric FISH probes was used to identify extrachromosomal DNA, and the tool was able to classify 83 percent of these signals as representing circular ecDNA amplicons. Interestingly, some of these cases revealed the presence of concurrent extrachromosomal and intrachromosomal signals, suggesting that some ecDNA had reintegrated into the genome.
Read More »Q&A column
Q. What is the minimum cutoff value for total nucleated cells and red blood cells in body fluids after which we need to perform cytospin? Read answer. Q. We treat all elevated troponins as critical values that necessitate a phone call to the ordering physician and documentation on the patient’s chart. Is this necessary? How does it affect patient treatment? Read answer.
Read More »Newsbytes
October 2020—Many prolific Twitter users describe the social media site as a time sink, but Andrew Schaumberg, PhD, begs to differ. After observing pathologists turn to Twitter to seek advice about difficult patient cases, he developed Pathobot, a free, artificial intelligence-driven search tool on Twitter that is designed to help pathologists connect with colleagues faster.
Read More »Put It on the Board
October 2020—Siemens Healthineers will collaborate with the Centers for Disease Control and Prevention and the Joint Research Centre of the European Commission on a research project to develop a novel process for standardizing SARS-CoV-2 assays.
Read More »Compass points chart the pandemic
September 2020—Between a rock and a hard place. Trying to stay ahead, trying to build inventory. Chasing multiple new testing requests. Anticipating influenza. That’s where laboratory leaders said their labs were in early August when CAP TODAY publisher Bob McGonnagle convened members of the Compass Group on Zoom to share their pandemic experiences. They shared surprise, too, that the situation is what it is: “Not a clue in my mind that this would go past the springtime,” said Stan Schofield, president of NorDx and senior VP, MaineHealth. McGonnagle asked them about the diversion of supplies, the coming flu season, IT support, lessons and long-term changes, and more.
See current issue below for additional COVID-19 coverage or access all COVID-19 articles here.
Real-time QC: on course for prime time?
September 2020—Bill Gates was just 10 years old and the Beatles were still playing live concerts when the concept of patient-based real-time quality control was proposed in 1965. At the time, patient-based real-time QC (PBRTQC) was based on the “average of normals,” a precursor of moving averages.
Read More »Oh, the places you’ll go when flu season hits
September 2020—The twinned challenge of testing for SARS-CoV-2 and the upcoming influenza season has a bit of The Cat in the Hat energy running through it. How does one manage to keep Thing One and Thing Two from creating unmitigated chaos? Maybe one doesn’t, not completely. A pandemic-based flu season will by its very nature be protean.
Read More »What’s new in latest transfusion medicine checklist
September 2020—Strong quality management, patient safety, and conformity with regulations are at the heart of the new and revised requirements in the 2020 CAP accreditation program transfusion medicine checklist, released in June.
Read More »Juan Rosai, ‘master of the neoplastic universe’
September 2020—Great mentors, once passed, live on in the ideas, memories, and dreams of their students. For those like me, lucky enough to have been a student of Juan Rosai, MD, and to have heard him speak, the experience will never be forgotten.
Read More »Why do universal HRD testing in ovarian cancer?
September 2020—Genetic testing in ovarian cancer has a therapeutic implication that will aid in developing a treatment plan, and it is pathologists who should take the lead in creating the testing protocol, said Samuel Caughron, MD, pathologist, president, and CEO of MAWD Pathology Group, in a recent CAP TODAY webinar. Dr. Caughron explained the rationale for universal homologous recombination deficiency testing in patients with advanced ovarian cancer. The webinar, made possible by a medical sponsorship from AstraZeneca, is at <a href="https://www.captodayonline.com">www.captodayonline.com</a>.
Read More »Behind book on professionalism: ‘we can do better’
September 2020—Professionalism in Pathology and Laboratory Medicine is a new book now out from CAP Publications. It provides a basic understanding, educational and assessment tools, 105 cases specific to pathology and laboratory medicine, guidance in recognizing and addressing lapses in behavior, discussions on best practices and legal and ethical aspects, and much more. Ronald E. Domen, MD, of Penn State College of Medicine and Hershey Medical Center, is editor. His co-editors are Richard M. Conran, MD, PhD, JD, of Eastern Virginia Medical School; Robert D. Hoffman, MD, PhD, of Vanderbilt University School of Medicine; Cindy B. McCloskey, MD, of the University of Oklahoma Health Sciences Center; and Suzanne Zein-Eldin Powell, MD, of Houston Methodist Hospital.
Read More »Targeting immune signaling checkpoints in AML
September 2020—Acute myeloid leukemia was one of the first diseases for which T cells were incorporated into the therapeutic paradigm, in the form of allogeneic stem cell transplant and donor lymphocyte infusion. Why then are there no approved immune therapies, or more specifically checkpoint inhibitors, for this T-cell–sensitive disease?
Read More »AMP case report: Culture-negative endocarditis due to Tropheryma whipplei
September 2020—A 64-year-old male presented with worsening shortness of breath, dry cough, and bilateral leg edema. He had a history of diabetes mellitus type two, hypertension, seropositive rheumatoid arthritis, and tobacco and alcohol abuse. CT scan demonstrated bilateral pleural effusions, pulmonary edema, subsegmental atelectasis, mildly enlarged hilar lymph nodes, mild cardiomegaly with a small pericardial effusion, and liver cirrhosis with a liver nodule. A hepatitis panel demonstrated positive serology for hepatitis C virus infection.
Read More »From the President’s Desk: Painful cuts ahead
September 2020—Pathologists have been feeling the pain for months. The COVID-19 pandemic triggered furloughs and layoffs even as we ran a grueling race to implement and scale high-quality testing. And now looming cuts to pathologist physician payments threaten to make our situation even worse.
Read More »Clinical pathology selected abstracts
September 2020—The clinical features and immune responses of people infected with SARS-CoV-2 who are asymptomatic are under investigation since people without disease symptoms can unknowingly spread the virus. As of Aug. 3, there were 17,965,128 confirmed COVID-19 cases worldwide, 4,749,138 of which were in the United States. The majority of those with SARS-CoV-2 infection have mild to severe respiratory illness with fever, cough, and shortness of breath, which appears two to 14 days after exposure. The authors conducted a study in which they described the epidemiological and clinical characteristics, viral levels, and immune responses in 37 asymptomatic people to better understand the clinical features and immune responses of people who are infected with SARS-CoV-2 and asymptomatic. The 37 asymptomatic people, all in the Wanzhou district of China, were diagnosed with RT-PCR–confirmed SARS-CoV-2 infections but had no relevant clinical symptoms in the preceding 14 days or while quarantined at the government-designated hospital for centralized isolation in Wanzhou.
Read More »Anatomic pathology selected abstracts
September 2020—Identifying patients who respond to immune checkpoint blockade is a significant challenge in oncology. PD-L1 expression by immunohistochemistry is the diagnostic gold standard for patient selection, but it does not capture all patients who may respond to immune checkpoint blockade (ICB). Recent gene-expression studies of high-grade serous ovarian carcinoma have defined an immunoreactive molecular subtype that shows a measurable favorable difference in patient survival compared with nonimmunoreactive subtypes, but no studies have demonstrated its impact on predicting response to ICB. As a step toward establishing the predictive value of gene-expression classifiers in ICB, the authors assessed the relationship between PD-L1 IHC and molecular subtypes of ovarian epithelial cancer. They analyzed 93 tissue specimens from patients with stages III and IV disease and compared PD-L1 IHC with gene expression by Agilent microarrays using The Cancer Genome Atlas-defined subtypes.
Read More »Molecular pathology selected abstracts
September 2020—Whole genome methylation profiling is used to subclassify neuroepithelial tumors and soft tissue sarcomas. Extending its use to much more common cancers, such as prostate cancer, has the potential to benefit a large number of patients. Metastatic castration-resistant prostate cancer (mCRPC) is the incurable and lethal form of prostate cancer and consists of different subgroups with variable morphologies and genomic alterations. The emergence of distinct subtypes of mCRPC likely represents adaption of the cancer cells to treatment and the microenvironment. The authors conducted a study that integrated methylation profiling with genomic sequencing and RNA transcriptome analysis in 100 mCRPC tumors, yielding a comprehensive molecular profile of these metastatic tumors.
Read More »Pathology informatics selected abstracts
September 2020—Whole slide imaging has been available for clinical, research, and educational use for decades, with several digital pathology systems cleared by the FDA for primary diagnosis. However, widespread adoption of this technology for routine practice has been slow. Likely reasons for the slow uptick in employing whole slide imaging (WSI) for sign-out include the cost of these systems, their lack of interoperability with laboratory information systems, pathologist resistance to using this digital modality, and regulatory restrictions on remote sign-out imposed by the Clinical Laboratory Improvement Amendments (CLIA). However, the COVID-19 pandemic led the Trump administration, on March 26, to temporarily waive these CLIA regulations, giving pathologists the flexibility to sign out cases digitally from their homes.
Read More »Q&A column
Q. Is the evaluation of gene copies by RT-PCR or multiplex ligation-dependent probe amplification a qualitative or quantitative assay? Copy number analysis of genes or chromosomes determines a numerical value, with a normal autosomal count being two. However, an FDA-approved microarray test (CytoScan Dx assay, Thermo Fisher Scientific) is labeled as a qualitative assay for the detection of copy number variations. Read answer. Q. How does using sodium heparin, in an attempt to reduce EDTA-induced platelet clumps, affect the platelet count? Read answer. Q. How do you know whether thyroid-stimulating hormone isoforms have been measured in an assay when the TSH levels are very high and free T4 is considerably less than the reference interval (i.e. less than 50 percent of the reference interval)? Read answer.
Read More »Newsbytes
September 2020—While the SARS-CoV-2 outbreak has led many long-standing companies to zig instead of zag, it caused the computational and digital pathology startup Crosscope to switch gears in the midst of developing its first product.
Read More »Put It on the Board
Roche launches Preanalytical System, announces FDA OK for HER2 Dual ISH test as CDx
September 2020—Roche launched its Cobas Prime Preanalytical System to improve efficiency in molecular diagnostics laboratories. It is now commercially available in the United States and markets accepting the CE mark. The system is designed to automate all preanalytic steps and features cross-contamination control of samples. It has track-connectable modular configurations with one workflow for multiple sample types, end-to-end automation with predictable lab turnaround time, and IT integration with sample and test tracking.
The laboratory tests of pandemic summer
August 2020—In March, the COVID-19 pandemic came in like a lion—and has yet to leave, like a lamb or anything else. Instead, it roared through April and May in early hot spots like New York City and New Orleans. As lockdowns took hold, the cautious hope was that by summer the virus would be tamed (if not simply go away “like a miracle” or “as the heat comes in,” per several infamous predictions), giving health care providers a chance to exhale before a likely second wave in the fall. Instead, June and July saw other cities and states hit hard in turn, while many places that appeared to have flattened the curve were starting to see concerning upticks in cases.
Read More »Targeting microbiology lab efficiency with AI
August 2020—Bringing an automated culture plate reading system into the Hennepin County Medical Center microbiology laboratory was never a question of if but when. “We need artificial intelligence to help us with active decision-making processes in the lab,” says Glen Hansen, PhD.
Read More »In 2020 checklist, a ‘gentle push’ to next quality level
August 2020—For quality management in the laboratory, it’s not enough to have checks and balances. The checks and balances have to work to improve quality. That’s how Stephen J. Sarewitz, MD, vice chair of the CAP Checklists Committee, characterizes the changes to the quality management requirements in the 2020 laboratory general checklist, released in June.
Read More »Lab with Ebola experience: COVID more complicated
August 2020—If there’s one thing scarier to experience than COVID-19, it’s Ebola. Or so you might think. “Ebola was easier,” says Beverly Dickson, MD, medical director of the clinical laboratory at Texas Health Presbyterian Hospital Dallas.
Read More »Steps to verifying SARS-CoV-2 antibody assays and what’s known about protective immunity
August 2020—The CAP treats emergency use authorization assays similar to FDA-cleared assays and thus requires full verification. In a June 4 CAP webinar, Neil Anderson, MD, D(ABMM), assistant director of clinical microbiology, Washington University School of Medicine in St. Louis, walked through how to approach verification for SARS-CoV-2 assays. Co-presenter Elitza Theel, PhD, D(ABMM), director of the infectious diseases serology laboratory at Mayo Clinic, reported what’s known about protective immunity against SARS-CoV-2.
Read More »Tumor budding assessment in CRC: why and how
August 2020—Tumor budding is a robust prognostic marker that should be reported at least in pT1 and stage II colorectal carcinomas and taken into account with other risk factors. Further evidence is needed for tumor budding assessment in specimens taken after neoadjuvant therapy, says Heather Dawson, MD, senior staff GI pathologist at the Institute of Pathology, University of Bern in Switzerland.
Read More »AMP case report: Burkitt-like lymphoma with 11q aberration
August 2020—Burkitt-like lymphoma with 11q aberration (BLL-11q) is a new provisional entity in the revised 2016 WHO classification of hematopoietic and lymphoid tumors.1 It refers to a subset of high-grade B-cell lymphomas that resemble Burkitt lymphoma with similar morphology, phenotype, and gene expression profiling, but lack MYC gene rearrangements. Instead, these lymphomas carry chromosome 11q proximal gains and telomeric losses, suggesting co-dysregulation of oncogenes and tumor suppressor genes.
Read More »COVID testing capacity falls short as flu season nears
August 2020—As the need for COVID-19 testing grows well beyond that for hospital patients, clinical laboratories in mid-summer were again overwhelmed by demand while at the same time bracing for flu season. That was the gist of a July 10 webinar that brought together Gyorgy Abel, MD, PhD, medical director of clinical chemistry, molecular diagnostics, immunology, and point-of-care testing at Lahey Hospital and Medical Center, Burlington, Mass.; Bob McGonnagle, CAP TODAY publisher; and moderator Steve Beuchaw, director of life science and medical device research, Wolfe Research.
Read More »Cytopathology in focus: Abnormal cervical screening tests: a personal approach
August 2020—If the past decade was directed toward aligning medicine with a personalized approach to therapy, this decade should further realize the implementation of health care decisions tailored to the patient. The updated 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors take a large step in that direction by relying on the input of personal data into a free online application that provides suggested management planning based on patient history and prior Pap/HPV test results.
Read More »Cytopathology in focus: New direction for thoracic small biopsy, cytology specimens
August 2020—Cytopathologists are keenly aware of the need to collect adequate cytologic tissue not only to arrive at a diagnosis but also to provide sufficient material for predictive and prognostic markers. This is especially true in the realm of non-small cell lung cancer, where biomarker testing is routinely used for the clinical management of patients with advanced-stage disease. The list of clinically relevant biomarkers in NSCLC is expanding. The most recent version of the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology includes MET exon 14 skipping mutations and RET as therapeutic targets for advanced NSCLC, in addition to the well-established EGFR and BRAF mutations, ALK and ROS1 rearrangements, and PD-L1 expression.
Read More »From the President’s Desk: Virtual meetings, for now
August 2020—In the past six months, many of us have developed video conferencing skills to an extent we never imagined we would need. Until the COVID-19 pandemic led to lockdowns in several states, a great deal of expertise in Zoom and other similar platforms was not a high priority among many pathologists. I am proud that our community adapted quickly and skillfully to continue providing the best care possible to our patients.
Read More »Clinical pathology selected abstracts
August 2020—Bacterial contamination of platelets continues to be an important cause of transfusion-associated morbidity and mortality. From 2001 to 2016, there were 51 deaths reported to the FDA due to transfusion of apheresis products contaminated with bacteria, including 30 deaths since testing for bacterial contamination was mandated in 2004. This mandate was implemented through primary culture of single-donor apheresis platelets in 2004 and then prestorage pooled platelets (PSPPs) in 2007. The authors conducted a study to compare the platelet bacterial contamination and septic transfusion rates before and after introducing testing of pooled and apheresis platelets by primary culture over an extended time period. They cultured platelet aliquots at issue and evaluated transfusion reactions.
Read More »Anatomic pathology selected abstracts
August 2020—The authors conducted a study in which they reviewed 354 cases of malignant diffuse mesothelioma in women from a database of 2,858 histologically confirmed cases. Pleural predominance was noted with 78 percent of pleural malignant mesotheliomas (MM) and 22 percent of peritoneal MM. The pleural tumors consisted of 72 percent epithelioid, 19 percent biphasic, and nine percent sarcomatoid variant. The peritoneal tumors consisted of 82 percent epithelioid, 13 percent biphasic, and five percent sarcomatoid. The immunohistochemical profile was typical of what is well accepted and previously described for MM.
Read More »Molecular pathology selected abstracts
August 2020—Patients and families suspected of having Mendelian diseases, syndromes thought to be caused by a single mutated gene, often undergo comprehensive next-generation sequencing to determine the underlying pathogenic variant. Whole exome sequencing, in which the coding regions of all genes are analyzed, is usually the preferred testing method. However, this identifies the diagnostic pathogenic variant in only 25 to 52 percent of cases. Whole genome sequencing appears to confer only marginal benefit over whole exome sequencing, presumably because the pathogenic variants likely are not missed by whole exome sequencing but may be misinterpreted as nondiagnostic. Among the variants that are difficult to interpret are those that affect RNA by directly influencing transcription, or altering normal splicing, or by mediating their effects through chromatin.
Read More »Q&A column
Q. If a person died from an overdose, would the toxicology screen always show which drugs were in their system? Read answer. Q. We are looking into using a scale to measure 24-hour urine samples, but we can’t find much literature about it. Is the variation between the measurement from a scale and actual volume clinically significant? What kind of validation is recommended? Read answer.
Read More »Newsbytes
August 2020—At Sonora Quest Laboratories, working backward has been a key strategy for leaping forward. Little by little, the Arizona-based integrated laboratory system has been retracing paper trails and assessing established processes as part of an ambitious plan to eliminate paper use across its seven commercial laboratories, 28 hospital labs, and more than 75 patient service centers.
Read More »Put It on the Board
August 2020—SpIntellx and CellNetix Pathology & Laboratories will collaborate to validate the SpIntellx HistoMapr-Breast Platform. The platform is the first in the companies’ larger efforts to use explainable artificial intelligence-assisted lab processes in the development of diagnostics, prognostics, therapeutic strategies, and drug development for tumor and non-tumor diseases.
Read More »Published in June:
At the pandemic’s serologic frontier
 The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves. That also meant months, possibly stretching into summer, in which laboratories faced more questions than answers, at a time when the demand for serology tests seems insatiable (and somewhat perplexing).
The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves. That also meant months, possibly stretching into summer, in which laboratories faced more questions than answers, at a time when the demand for serology tests seems insatiable (and somewhat perplexing).
A preanalytical Rx for fallible GDM testing
July 2020—Gestational diabetes mellitus, if left untreated, is notoriously dangerous for mothers and their babies, making timely diagnosis critical. Yet the disease is similarly well known for being chronically under-diagnosed by laboratory testing.
Read More »New checklist hones lab’s verification and validation requirements
July 2020—If a manufacturer assists a laboratory in setting up a new FDA-approved or -cleared test, the lab must make sure that the personnel who will perform the test participate in the verification or validation study.
Read More »Outbreak detection of novel pathogens: Is AI the answer?
July 2020—A machine learning algorithm, used in conjunction with BioFire’s Syndromic Trends, demonstrated a mechanism for near real-time outbreak detection of enterovirus D68, says a study reported in the Journal of Clinical Virology.
Read More »In CRC, distinguishing tumor deposit from lymph node
July 2020—When patients who have colorectal cancer surgery at another institution seek further care at Beth Israel Deaconess Medical Center in Boston, the Beth Israel pathologists routinely request the original slides. Raul S. Gonzalez, MD, a gastrointestinal pathologist at Beth Israel, says he usually agrees with everything the outside pathologist reports. But if there are differences, lymph nodes versus tumor deposits is one place where he might disagree.
Read More »Closing the workflow loop: HistoQC for digital slides
July 2020—Unveiled in 2018, HistoQC, an open-source quality control tool for digital pathology slides, was an “awakening to a problem” and the kickoff of a conversation, says Andrew Janowczyk, PhD, its main investigator. And while it’s hard to measure the tool’s use because it’s freely available, he says, reception has been strong. “It’s been a pretty good ride,” he says of its first two years.
Read More »AMP case report: Unexpected diagnosis of indolent systemic mastocytosis through evaluation of NGS data
July 2020—A 77-year-old woman presented at our institution for care with a remote history of lymphoma. Three years earlier she had a bone marrow biopsy performed that by report showed five percent monoclonal kappa restricted plasma cells. At the initial presentation at our institution, serum protein electrophoresis and immunofixation showed an IgA kappa paraprotein (0.52 g/dL), and bone marrow biopsy showed less than five percent plasma cells by CD138 immunohistochemistry that were kappa light chain restricted by flow cytometry, with normal renal function and calcium level. A PET-CT indicated no lytic bone lesions. These findings confirmed the diagnosis of IgA kappa monoclonal gammopathy of undetermined significance (MGUS). Her past medical history was remarkable for chronic oral ulcers for five years of possible autoimmune etiology that were responsive to methylprednisolone, a stroke, hypertension, and hyperlipidemia.
Read More »At UW, anatomic pathology rotation moves online
July—When COVID-19 set in, much of residency education in the U.S. moved online. At the University of Washington School of Medicine, anatomic pathology faculty took online learning a step further by creating a virtual two-week anatomic pathology rotation for medical students. The faculty is aiming for a four-week virtual rotation inclusive of more laboratory medicine, to be used even after the pandemic has passed.
Read More »A panel’s take on instruments, connectivity, COVID
July 2020—Has the pandemic changed your thinking or that of your customers? That’s one of the questions CAP TODAY publisher Bob McGonnagle put to seven representatives of five companies and two other panelists in a May 13 roundtable on chemistry/immunoassay analyzers and testing. But first up were other topics: scalability, connectivity, standardizing platforms across health systems, consistent sourcing of antibodies, and open automation.
From the President’s Desk: Remembering Gene Herbek
July 2020—It is with profound sadness that I write about the passing of a dear friend. Gene Herbek, MD, served as president of the CAP from 2013 to 2015, and many CAP TODAY readers will remember his informative column in these pages. For details about his life and accomplishments, please read his obituary in this issue (page 12). I’d like to spend this column focused on how he shaped the CAP, and what an honor it was to have known him.
Read More »In memoriam: Gene N. Herbek, MD 1949 –2020
July 2020— Gene N. Herbek, MD, CAP president from 2013 to 2015, died June 4 after a brief illness. He was a CAP governor from 1998 to 2004 and secretary-treasurer from 2006 to 2011. Dr. Herbek was medical director of the Methodist Women’s Hospital laboratory and medical director of transfusion services for The Pathology Center at Methodist Hospital, Omaha, Neb. He chaired multiple CAP groups over the years, among them the councils on Public Affairs and Membership and Public Affairs and the Finance, Credentials, Nominating, Political Action, and Insurance committees.
Read More »Clinical pathology selected abstracts
July 2020—The College of American Pathologists launched the Pathology and Laboratory Quality Center for Evidence-Based Guidelines in 2009 to develop and promote laboratory practice guidelines (LPGs) using the National Academy of Medicine’s (NAM) standards for developing trustworthy guidelines. The center has published 17 evidence-based LPGs, including updated versions, using NAM’s criteria. In 2013, the CAP was awarded a five-year cooperative agreement grant from the Centers for Disease Control and Prevention to increase the effectiveness of its LPGs. The intent of the agreement was to assess awareness and adoption of two CAP LPGs: IHC assay validation (IHC VAL) and initial workup of acute leukemia (AL).
Read More »Anatomic pathology selected abstracts
July 2020—Smooth muscle tumors represent the second most common mural mesenchymal neaoplasm in the gastrointestinal tract, yet established criteria for prognostically assessing these tumors are lacking. The authors conducted a study on a large cohort of surgically resected intramural gastrointestinal smooth muscle tumors from 31 institutions to identify potential prognostic features. At each location, expert gastrointestinal or soft tissue pathologists, or both, assessed pathologic features. IHC confirmation was required.
Read More »Molecular pathology selected abstracts
July 2020—Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DiHS/DRESS) is a rare but well-known full-body inflammatory skin condition that leads to life-threatening complications if untreated. The authors conducted a case study that involved a 44-year-old male who developed DiHS/DRESS after taking the combined antibiotic trimethoprim-sulfamethoxazole. Following standard treatment for this condition, the patient was started on high-dose prednisone, which provided no benefit. Subsequent tapering of prednisone advanced the disease to a toxic epidermal necrolysis-like condition. The patient was then given etanercept and high-dose intravenous immunoglobulin, without benefit. Next, the patient received cyclosporine, which resulted in some improvement but led to uncontrollable renal hypertension.
Read More »Q&A column
Q. Is it acceptable for a clinical laboratory to calculate ionized (free) calcium if calcium ion-selective electrode is not available? Are results of calculated ionized (free) calcium of acceptable accuracy in clinical practice? And what is the recommended formula for performing this calculation? Read answer. Q. Under checklist requirement COM.04250 “Comparability of Instruments and Methods —Nonwaived Testing,” what is the minimum number of samples that should be analyzed and which acceptance criteria should be used for the comparison? In addition, what parameters in the complete blood count do not apply for comparison purposes? Read answer. Q. I read an article about phlebotomy that stated for proper patient care, the recommended maximum number of blood draw attempts is four. The hospital at which I work recently implemented a procedure in which the nurses perform blood draws, instead of laboratory personnel. The procedure requires a nurse to attempt a blood draw twice and, if not successful, ask another nurse, and then ask the nursing supervisor before finally calling the laboratory. This policy has been difficult for hospital staff, and I feel terrible for patients who get stuck numerous times. Can you provide feedback that I can use to express my concerns to hospital administration? Read answer.
Read More »Newsbytes
July—In the early days of the COVID-19 pandemic, when the University of California, Berkeley's Innovative Genomics Institute decided to rapidly shift gears from conducting research to testing the community for SARS-CoV-2, some insiders may have thought the university was biting off more than it could chew under a tight timeframe. Yet a look at the lab 23 days later surely had any doubters eating their words.
Read More »Put It on the Board
AMP recommends PGx alleles for genotyping testing for warfarin response July 2020—The Association for Molecular Pathology published in May consensus, evidence-based recommendations to aid in the design, validation, and interpretation of clinical genotyping tests for the prediction of warfarin response. The new guideline on clinical warfarin sensitivity genotyping allele selection completes a series of three reports intended to facilitate testing ...
Read More »At the pandemic’s serologic frontier
June 2020—The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves.
Read More »Published in June: Autopsies show many faces of COVID-19 Sudden” and “global” are descriptors that seldom appear in tandem, especially in relation to disease epidemiology. But they both fit the COVID-19 pandemic, which has left the health care world reeling. “COVID-19 has infected the entire planet pretty much all at once,” says Alex K. Williamson, MD. Read more.
Read More »HIV, TB requirements in latest accreditation checklist edition
June 2020—Best practices for HIV primary diagnostic testing and rapid detection of Mycobacterium tuberculosis complex are clarified and codified in new checklist requirements in the 2020 Laboratory Accreditation Program checklist edition published June 4.
Read More »ER, PgR, HER2 expression rates seen in Q-Probes
June 2020—With release of the latest Q-Probes study, titled “Expression Rates in Invasive Breast Carcinoma,” the CAP Quality Practices Committee fills a gap by providing data collected from a diverse set of 21 U.S. laboratories on the average frequency of various ER, PgR, and HER2 expression results.
Read More »Amid COVID-19 crisis, pathologists fill a critical gap
June 2020—At NYU Langone Health, pathologists and others typically not seen out front in the fight against COVID-19 became the bridge between families and the floors. When Katherine A. Hochman, MD, associate chair for quality in the Department of Medicine at NYU Langone, contracted a mild case of COVID-19, she finally had a chance to take a step back and think. Before going into quarantine to recover, Dr. Hochman had been on the floors day in and day out attending to COVID-19 patients.
Read More »Predicting response to therapy with BH3 profiling
June 2020—Precision medicine in oncology, which today is nearly universally about genetics, needs to move beyond omics and static approaches, Anthony Letai, MD, PhD, professor of medicine at Dana-Farber Cancer Institute and Harvard Medical School, said at last year’s meeting of the Association for Molecular Pathology. Dr. Letai reported how his laboratory uses dynamic BH3 profiling, a novel assay that detects BCL2 protein dependence in cancer cells and measures changes in their apoptotic priming, to predict clinical response to therapy. He and others call it functional precision medicine.
Read More »In NSCLC, biomarker testing rates fall short
June 2020—Testing rates for actionable biomarkers in metastatic non-small cell lung cancer patients are below where they should be, and the overlap of PD-L1 expression with genomic targets causes confusion for oncologists and patients, said Geoffrey R. Oxnard, MD, oncologist at Dana-Farber Cancer Institute and associate professor of medicine at Harvard Medical School, in a recent CAP TODAY webinar.
Read More »AMP case report: CCND1/IGH fusion amplification in a case of plasma cell myeloma
June 2020—A middle-aged adult presented with shortness of breath and bruising and was found to have leukopenia (WBC 4.16 K/mcL; normal range 4.50–11.00 K/mcL) and anemia (Hgb 7.6 g/dL; normal range 12–16 g/dL) with rouleaux. The hypercellular bone marrow core biopsy (80–90 percent cellularity) contained 90 percent plasma cells of variable morphology, some with prominent nucleoli and occasional binucleate forms.
Read More »In Italy, lessons learned for lab testing
June 2020—The key lesson for policymakers and hospital administrators stemming from the pandemic is that continuing to cut human and economic resources will create large organizational issues when the entire system of care, including laboratory diagnostics, is challenged by “an enormously amplified volume of tests to manage emergent situations,” write Giuseppe Lippi, MD, of the University of Verona, and Mario Plebani, MD, of University Hospital of Padova, Italy, in an opinion paper published online March 19.
Read More »Letters
June 2020—I enjoyed the recent roundtable on billing and reimbursement (April 2020), along with the product guide on billing software. As we all know, since that time COVID-19 has taken center stage. Here are some thoughts and predictions to bring your readers up to date. Never before has a nation turned off a large percentage of its health care system overnight. This change has caused a train wreck-like boxcar effect that is rippling through medical billing operations for laboratories and pathology groups.
Read More »Published in July:
Reading COVID-19’s signature: lung tissue injury
 Alain Charles Borczuk, MD, began his practice of pathology as a resident 28 years ago and has spent quite a bit of his career doing autopsies. But it is this year, during the pandemic, that he’s finding some of the best applications of his autopsy work as he seeks to understand the lung injury patterns in SARS CoV-2, or COVID-19 patients. Read more.
Alain Charles Borczuk, MD, began his practice of pathology as a resident 28 years ago and has spent quite a bit of his career doing autopsies. But it is this year, during the pandemic, that he’s finding some of the best applications of his autopsy work as he seeks to understand the lung injury patterns in SARS CoV-2, or COVID-19 patients. Read more.
From the President’s Desk: Getting through the pandemic
June 2020—Like so many CAP TODAY readers, I have been spending the vast majority of my time working on COVID-19. In Georgia we have the dubious distinction of having a county with one of the highest per capita death rates due to COVID-19 in the country—at least as of early May when I wrote this. Things are moving so fast with this pandemic that by the time you read this, much will have changed.
Read More »Clinical Pathology Selected Abstracts
June 2020—More than 1.7 million new diagnoses of cancer occur in the United States each year, and they are almost exclusively made by pathologists who evaluate patient specimens and issue a written diagnostic report. These patients often are not given the opportunity to talk with the pathologist who made the diagnosis or view their tissue through a microscope. There is little published data on patient-pathologist consultation programs in which patients can review their reports and slides with the pathologist. In addition, the number of patients who may be interested in this service is not known. The authors conducted a study to quantify patients’ interest in patient-pathologist consultation programs and qualitatively analyze their motivations for interest or disinterest.
Read More »Anatomic Pathology Selected Abstracts
June 2020—The authors conducted a study in which they independently evaluated the utility and prognostic value of tumor budding according to International Tumour Budding Consensus Conference (ITBCC) criteria in a large well-characterized European gastric cancer cohort. They assessed tumor budding according to the ITBCC criteria for 456 consecutive, surgically treated gastric cancers using the scoring system Bd0 (no buds), Bd1 (one to four buds), Bd2 (five to nine buds), and Bd3 (10 or more buds). Cases with tumor budding were divided into low-budding (Bd1/Bd2) and high-budding (Bd3) groups. The tumor budding score was analyzed in relation to clinicopathological parameters, overall survival, and tumor-specific survival. The authors found that 115 (25.2 percent) cases had no tumor budding, 104 (22.8 percent) had low tumor budding, and 237 (52 percent) had high tumor budding.
Read More »Molecular Pathology Selected Abstracts
June 2020—The COVID-19 pandemic has focused the world’s attention on using sensitive high-throughput molecular diagnostic testing for the SARS-CoV-2 virus as a public health tool for “flattening the curve” of the infection. Although initial shortages of specialized polymerase chain reaction (PCR) testing reagents that plagued the early weeks of the pandemic have slowly improved (as of CAP TODAY press time), an obstacle to universal testing continues to be the first-step bottleneck of collecting respiratory tract samples for virus-specific reverse transcriptase-PCR (RT-PCR) testing. The traditional gold standard sample for COVID-19 testing has been a nasopharyngeal (NP) swab. However, nationwide shortages of NP swabs, personal protective equipment (PPE), and viral transport media have intermittently delayed the testing process. In an attempt to alleviate these critical sample-collection issues and promote more widespread testing, the medical community and other entities have been investigating alternative sample-collection procedures.
Read More »Q&A column
Q. Are there high-specificity IHC stains for diagnosing mesothelioma that differ from those recommended in the “Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group”? Read answer. Q. For our hospital tissue and blood committee, we would like to expand the tissue component to look at more preoperative versus postoperative diagnoses (acute appendicitis, for example) and anything else that should be evaluated, such as adequacy of tissue and blood samples. Is there a resource that provides a list of tissue-related quality metrics that we can evaluate? Read answer.
Read More »Newsbytes
June 2020—The global market for health care chatbots has been growing at a fairly rapid pace in recent years, but “COVID-19 is the thing that’s going to make chatbots mainstream,” says Greg Kefer, chief marketing officer at the chatbot company LifeLink Health. A 2019 Allied Market Research report, released just months prior to the COVID-19 pandemic, projected the health care chatbot industry would reach $345.3 million by 2026, a steep rise from 2018, when it garnered $116.9 million (www.alliedmarketresearch.com/healthcare-chatbots-market). But now, the COVID-19 pandemic has put into sharp relief one of the key value propositions of chatbots—unlimited scale, which means the timeline for adoption “just got massively compressed,” says Kefer, whose software-as-a-service company develops enterprise-level chatbots for large health care organizations.
Read More »Put It on the Board
Better cancer diagnostics at core of Future of Pathology report June 2020—Leica Biosystems launched in April The Future of Pathology Expert Report written by an international panel of pathologists in consultation with health care executives, cancer stakeholders, and pathology leaders. In the report, each member of the panel—Tiffany Graham, MD, and Jerad Gardner, MD, in the U.S., and Bethany Williams, MBBS, PhD, and Matthew Clarke, MBBS, in the U.K.—addresses one of four subjects: pathology education, digital pathology and artificial intelligence, improving perceptions of pathology, and molecular pathology. The theme of the report (available at www.thefutureofpathology.com) is improving and transforming cancer diagnostics.
Read More »A lab world embroiled in pandemic
May 2020—Along with SARS-CoV-2, clinical laboratory testing has been hiding in plain sight far longer than many people realize. But it took the novel coronavirus (which, frankly, hardly feels novel anymore) to make that clear to the rest of the world. As the COVID-19 pandemic spread across the globe, laboratory testing crashed the news cycle. National leaders sought to reassure citizens by promising millions of test kits.
Read More »Adapting test ordering to novel tick-borne disease rise
May 2020—When tick-borne infections are the topic of discussion, talk of Lyme disease, caused by Borrelia burgdorferi, has tended to predominate. But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks has been climbing as well.
Read More »Suiting up for COVID-19 autopsies, sharing findings
May 2020—To combat the spread of COVID-19, Louisiana’s governor in late March issued an order directing state residents to limit their excursions outside.
Read More »AI roundtable: hopes, hurdles, hype vs. reality
Michael Becich, MD, PhD, of the University of Pittsburgh School of Medicine, and five others spoke with CAP TODAY publisher Bob McGonnagle in March about the hype versus reality of artificial intelligence and the tension around it. Here is what they had to say.
A conversation: Specimen collection and testing for SARS-CoV-2
May 2020—But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks—including non-Lyme bacteria, protozoa, and viruses—has been climbing as well.
Read More »In memoriam: William B. Zeiler, MD 1921–2020
May 2020—William B. Zeiler, MD, CAP president from 1987 to 1989, died March 24 at age 99. Dr. Zeiler was CAP vice president from 1985 to 1987 and a member of the Board of Governors from 1981 to 1985. He led and was a member of several CAP councils, committees, and commissions. He was named CAP Pathologist of the Year in 1990, and winner of the Gold Headed Cane Award from the World Association of Societies of Pathology and Laboratory Medicine in 2001.
Read More »From single to syndromic tests for SARS-CoV-2
May 2020—Christine C. Ginocchio, PhD, MT(ASCP), in an April 8 CAP TODAY webinar, reported findings on the analytical efficiencies and sensitivities of four of the original and most common of the SARS-CoV-2 assays. Read more.
Read More »AMP case report: NGS as the tiebreaker in tumors with similar morphology and equivocal immunophenotype
May 2020—Traditionally, histopathologic diagnosis has been regarded as the gold standard for most disease processes including cancer. However, in certain circumstances, a final histopathologic diagnosis cannot be rendered despite extensive conventional ancillary testing such as immunohistochemistry. In recent years, molecular testing has revealed specific variant signatures for many tumors, which can be used to determine a final diagnosis.
Read More »Study of inpatient test utilization practices set to begin
May 2020—Like a top 40 radio hit, test utilization is a topic that can sometimes seem to be overplayed. But the COVID-19 pandemic brings into sharp relief its importance. “What we’ve seen is organizations that have more mature test utilization efforts in place may be better able to handle these crises,” says Peter L. Perrotta, MD, professor and chair of pathology, anatomy, and laboratory medicine at West Virginia University School of Medicine and director of pathology services, West Virginia University Health System.
Read More »Cytopathology in focus: Direct HPV testing in FNAs from cervical lymph node metastases
May 2020—According to a Centers for Disease Control and Prevention study from 2008 to 2012, there are about 16,000 cases of HPV-positive oropharyngeal squamous cell carcinoma per year in the United States. These carcinomas tend to present with small primary lesion and early nodal metastases as an initial manifestation of the disease. Furthermore, carcinoma of unknown primary presenting as a cystic metastasis in the head and neck has been linked frequently to oropharyngeal squamous cell carcinomas, mainly of palatine tonsils and base of the tongue.
Read More »Cytopathology in focus: Gynecologic cytology PT appeals: where they started,where they stand
May 2020—The CAP implemented proficiency testing for cervical cytology in 2006 as mandated by federal legislation. The performance of participants and granting of appeals on glass slides in the first year of the CAP Pap PT program was reported in detail by Crothers, et al. Once a participant initiates an appeal, the slide in question is pulled from the program for a blinded review by three board-certified anatomic pathologists who are members of the CAP Cytopathology Committee. In the first year, 155 participants failed the PT examination and appealed their testing results on 86 individual slides. After review, appeals were granted for 21 slides, resulting in 45 exam failure reversals. The overall appeal rate was 13/1,000 slides in the program.
Read More »Cytopathology in focus: Can you send us your cytology slides? Labs are reimbursed for slides accepted into programs
May 2020—The CAP relies on the generous submission of slide-based cases from laboratories to maintain the excellent quality of its Cytopathology Educational Programs in Gynecologic Cytopathology (Pap Education), Non-Gynecologic Cytopathology (NGC), Fine Needle Aspiration Cytopathology (FNA), and Proficiency Testing Program in Gynecologic Cytopathology (Pap PT). The CAP Cytopathology Committee, composed of 26 pathologists, two junior members, and two cytotechnologist members, meets quarterly and members submit glass slides to these varied programs.
Read More »Shorts on Standards: ISO 22367: Application of risk management to medical laboratories
May 2020—Hazard. Harm. Risk. Benefit. Performing any activity poses risks. The laboratory deals with hazards known and understood, or unknown and unanticipated, that can lead to harm. Laboratorians attempt to minimize these risks to the point where benefit exceeds residual risk that cannot be further minimized. We understand these concepts and terms and deal with them in everything we do. For optimal patient care and for the safety of our employees, a formal approach to risk assessment is desirable to identify pertinent risks, assess those risks, determine if and when the risk has been sufficiently minimized or if mitigation measures are worth the benefit we expect to see, and document each step.
Read More »Put it on the Board
Clinical research registry aims to answer crucial COVID-19 questions May 2020—A Healthcare Worker Exposure Response and Outcomes Registry was launched April 13 to unite health care workers in the U.S. for rapid-cycle research. The goals of the so-called HERO registry are to engage workers in a research community, understand their experiences, and track outcomes associated with caring for patients with ...
Read More »From the President’s Desk: Heroes in the COVID-19 pandemic
May 2020—The most pressing topic in pathology today is the COVID-19 pandemic. Because this column must be written a few weeks before it is printed and delivered to readers, anything I write in April will be out of date by the time you see it. Numbers will be wrong, and comments on testing methodology will be obsolete. Instead, I’ll focus on the bigger picture. As we watch the world’s response to the pandemic, I am proud to be a physician and a pathologist. I am honored to work with phenomenal laboratory personnel, and this is by no means unique to me. Across the medical community, we have seen people come together for a shared mission in ways we have never experienced before. The demands on our laboratories are tremendous, and we are rising to the challenges of producing tests and directing therapies under conditions we have not seen before.
Read More »Newsbytes
May 2020—Imagine the potential educational benefits of pathology residents being able to see the precise path that the eyes of experienced pathologists take as they scan a whole slide image. Preliminary research has suggested that showing residents visual representations of a pathologist’s eye-tracking movement overlaid over a whole slide image can impact how they learn, says Sharon E. Fox, MD, PhD, a pathologist at the Southeast Louisiana Veterans Healthcare System and associate director of research and development, Department of Pathology, Louisiana State University Health Sciences Center.
Read More »Clinical Pathology Selected Abstracts
May 2020—Laboratory costs represent approximately three to four percent of overall health care expenses but drive 70 to 80 percent of decisions made by physicians. One way to control laboratory testing expenditures is through appropriate utilization. For example, thyroid and antinuclear antibody (ANA) testing have reliable initial screening tests, yet specialized testing is overutilized for both. In 2000 guidelines, the College of American Pathologists and American College of Rheumatology established that the ANA screen by immunofluorescence in the setting of a negative result is sufficiently sensitive not to perform further testing with subserologies and that thyroid-stimulating hormone (TSH) is a sensitive marker of thyroid function and can often be used without further testing with a normal TSH.
Read More »Q&A column
Q. Can you provide a current reference for the various modifications of transfused red blood cells — for example, irradiation and white cell depletion — and their indications? Read answer. Q. In the context of allowable error relative to the immature platelet fraction test, is the analytical measurement range applicable to the IPF? Read answer. Q. What is the normal random plasma glucose value? Read answer.
Read More »Anatomic Pathology Selected Abstracts
May 2020—Flat epithelial atypia is an alteration of terminal duct lobular units by a proliferation of ductal epithelium with low-grade atypia. No consensus exists regarding whether the diagnosis of flat epithelial atypia in core needle biopsy necessitates excision. The authors retrospectively identified all in-house core needle biopsies with flat epithelial atypia obtained at their institution between January 2012 and July 2018. They reviewed all core needle biopsy slides and assessed radiologic-pathologic concordance. An upgrade was defined as invasive carcinoma or ductal carcinoma in situ in the excision, or both. The excision slides of all upgraded cases were re-reviewed.
Read More »Molecular Pathology Selected Abstracts
May 2020—Each year a growing number of novel viruses are discovered in the wild animal kingdom with the use of next-generation sequencing technology. However, the current method of functionally assessing the zoonotic potential—that is, the potential to be transmitted to humans—of novel viruses involves synthesis of viral genomes (tens of thousands of bases) and reverse genetic engineering to produce a recombinant virus. This is expensive and time-consuming, and it is not practical due to the scale of new viral strains being discovered. To overcome this hurdle, Letko, et al., developed a rapid and cost-effective method that could functionally assess a number of related viruses for zoonotic potential. The essential component of viral cross-species transmission is cell entry, which is a multi-step process involving attachment of the virus to the host cell surface, receptor engagement, processing of host proteases, and downstream membrane fusion.
Read More »AST breakpoints: a case of not aging gracefully
April 2020—When Romney Humphries, PhD, D(ABMM), was section chief for clinical microbiology at UCLA Medical Center, it wasn’t unusual for her to take calls from worried clinicians who were concerned about patients who’d been transferred to the hospital for a higher level of care. The accompanying laboratory reports would indicate the presence of an isolate that was susceptible to a particular drug. But when Dr. Humphries’ lab did its own testing, the results were strikingly different: The isolate was drug resistant. When Dr. Humphries would call the first lab, the reason for the discrepancy became clear. “Sure enough, they were using old breakpoints,” says Dr. Humphries, who is now chief scientific officer, Accelerate Diagnostics, and professor of pathology, University of Arizona. Unlike Bordeaux, antimicrobial susceptibility test breakpoints do not age well. Over time, they might no longer be clinically useful, even as they continue to be used clinically. Dr. Humphries’ anecdote is, of course, just that—an anecdote.
Read More »ER/PgR guideline hones approach to ER-low positives
April 2020—The CAP and the American Society of Clinical Oncology released two years ago a focused update of their clinical practice guideline for HER2 testing for breast cancer, following an update in 2013. Read more.
Read More »Moving beyond immunoassays for poisoned patients
April 2020—Bypassing immunoassays as the first step in toxicology testing can minimize clinician calls to the laboratory about negative toxicology reports for apparently overdosed patients and save diagnostic time. Read more.
Read More »Transfusion cases out of cabinet, into new book
April 2020—CAP Publications released this month a new book titled Transfusion Medicine: A Compendium of Educational Cases, from the CAP Transfusion, Apheresis, and Cellular Therapy Committee. In it are 20 cases, each with a history, discussion, and questions and answers. Read more.
Read More »Billing practices, problems — we ask the experts
April 2020—Billing and collecting for pathology and laboratory services is “tough and getting tougher.” That’s the view of consultant Al Sirmon of Pathology Practice Advisors, Pawleys Island, SC. “In what other industry,” he asks, “do you rely on a third party to decide how much and when you’re going to get paid for a service you provide to someone else?” On the bright side, he says: “We have better tools now.”
PD-L1 testing in triple-negative breast cancer: Post hoc IMpassion130 substudy evaluates PD-L1 IHC assay performance
April 2020—IMpassion130 was the first phase three trial to demonstrate a clinical benefit of cancer immunotherapy in patients with PD-L1-positive metastatic triple-negative breast cancer, and based on the data, atezolizumab plus nab-paclitaxel is approved for this indication. In the trial, the Ventana SP142 PD-L1 assay with a one percent or greater cutoff was used to evaluate PD-L1 expression in immune cells. But questions remained about how to best identify patients who could benefit from the drug combination, Hope S. Rugo, MD, professor of medicine and director of breast oncology and clinical trials education at the University of California San Francisco Comprehensive Cancer Center, said in a CAP TODAY webinar last November.
Read More »Student fellowship’s pluses seen in the field and out
April 2020—At Oregon Health and Science University, one of the oldest post-sophomore fellowship programs in pathology is recruiting steadily and channeling a quarter to a half of its student fellows into pathology residencies. Dating back to the 1920s, the OHSU Pathology Student Fellowship, as it is now known, starts recruiting at the end of the first year of medical school, says Nicole Andeen, MD, assistant professor of pathology and co-director of the program. “Medical students join after the preclinical curriculum or after a year in clinical rotations.” With former student fellows spreading the word, “recruitment happens naturally,” she says.
Read More »Q&A column
Q. For the population with diabetes, what can the clinical laboratory do to promote evidence-based testing and monitoring for chronic kidney disease? Read answer. Q. What are the proficiency testing enrollment requirements if an analyte is tested in multiple locations within the laboratory? Read answer. Q. With regard to specimens from oncology patients exhibiting hyperleukocytosis, the Beckman Coulter DxH 800 analyzer used by our laboratory autocorrects the RBCs, but the lab is still seeing errors on the indices (hemoglobin, MCV). What is the best way to correct for potential WBC interference on the RBC indices? Read answer.
Read More »Put It on the Board
Ortho Clinical Diagnostics in February made available its Vitros XT 3400 Chemistry System.
April 2020—Ortho Clinical Diagnostics in February made available its Vitros XT 3400 Chemistry System. The new system, like the Vitros XT 7600 Integrated System, simultaneously performs two tests frequently ordered together on one Vitros XT MicroSlide. Double assay processing offers a 25 percent faster turnaround time on a common panel of assays, Ortho said in its Feb. 26 statement, with an average processing time of 7.5 minutes. The XT MicroSlide allows for a sample volume of 2.7 μL.
From the President’s Desk: How the government influences your practice
April 2020—It is important for pathologists to understand just how much influence the federal government has on our practice of medicine. In last month’s column, I discussed ways in which the government dictates how and whether we are paid for our services. This month I’ll address the role of the federal government in determining where and how we practice. You probably already know that pathology labs are among the most highly regulated areas within health care, subject to regulations from multiple agencies.
Read More »Newsbytes
R programming language gains steam in pathology labs April 2020—Among laboratories focused on expanding data analytics, the statistical programming language R has a loyal user base that is steadily growing. “There is a crew of us that are really trying to show the utility of R for laboratories,” says Stephen Master, MD, PhD, chief of the Division of Laboratory Medicine and director of the Michael Palmieri Laboratory for Metabolic and Advanced Diagnostics at Children’s Hospital of Philadelphia.
Read More »Clinical Pathology Selected Abstracts
April 2020—An outbreak of pneumonia occurred in Wuhan, Hubei province, China, in December 2019. A novel coronavirus was identified as the causative agent and named SARS-CoV-2 by the World Health Organization (WHO). The disease, COVID-19, is considered a relative of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). SARS-CoV-2–infected patients presented with a dry cough, dyspnea, fever, and bilateral lung infiltrates.
Read More »Anatomic Pathology Selected Abstracts
April 2020—Lymphocytic esophagitis is a well-known manifestation of Crohn disease among children but is not considered an immune-mediated mucositis in adults. The authors conducted a study for which they hypothesized that adult-onset lymphocyte-predominant esophagitis is also an immune-mediated inflammatory pattern, the nature of which has been masked by other conditions that feature esophageal lymphocytosis and occur in older adults. The intent of the study was to consolidate diagnostic criteria for lymphocyte-predominant esophagitis and determine its clinical significance.
Read More »Molecular Pathology Selected Abstracts
April 2020—Prior to the recent novel coronavirus outbreak, the vast majority of coronaviruses that were known to be pathogenic in humans caused mild symptoms, with the exception of two strains. These included SARS (severe acute respiratory syndrome coronavirus [SARS-CoV]) and MERS (Middle East respiratory syndrome coronavirus [MERS-CoV]). SARS was documented as having emerged in the Guangdong province in southern China in 2002 and having caused at least 774 reported fatalities worldwide, while MERS, which was first detected in Saudi Arabia in 2012, was responsible for at least 858 fatalities worldwide.
Read More »Struggling to find a foothold with NAFLD
March 2020—For pathologists, a first look at nonalcoholic fatty liver disease can be jarring. Purva Gopal, MD, has seen her share of initial biopsies on patients whose “livers are already cirrhotic,” she says. So has Cynthia Guy, MD, professor, Department of Pathology, and chief of the liver and GI surgical pathology section, Duke University Health System. It’s not a good look, obviously, so it’s one she’s doing her best to share with clinical colleagues. At her institution, pathologists have built a strong connection with the hepatologists and gastroenterologists, she says, and NAFLD is part of the regular show-and-tell.
Read More »C. auris: ‘The more we see, the more resistance’
March 2020—While developing into a global health emergency that has killed thousands, the outbreak of the SARS-CoV-2 coronavirus identified in China riveted the public and raised awareness of infectious diseases and their perils.
Read More »For molecular ID testing, micro lab or molecular lab?
March 2020—Where should molecular infectious disease testing be performed? That was the question at the center of a point-counterpoint session at last year’s AMP annual meeting.
Read More »In CML, BCR-ABL monitoring takes on new importance
March 2020—Three decades ago, when Jerald Radich, MD, started doing BCR-ABL testing, the process was highly hands-on. In those days, “we used water baths to do PCR,” said Dr. Radich, an oncologist and director of the molecular oncology lab and member of the Clinical Research Division, Fred Hutchinson Cancer Research Center, in a recent CAP TODAY webinar.
Read More »Addressing the shortcomings of ANA testing by IFA
March 2020—Standardizing indirect immunofluorescence testing for antinuclear antibodies is a critical task for the laboratory community, and it’s more urgent now that new classification criteria make positive ANA a key factor in diagnosing lupus, said Mark H. Wener, MD, in a session at last year’s American Association for Clinical Chemistry annual meeting.
Read More »AMP case report: Advantages of SNP chromosomal microarray over conventional FISH and DNA tests for methylation-specific PCR-positive Prader-Willi syndrome
March 2020—Prader-Willi syndrome (PWS) is a rare genetic multisystem disorder with a reported incidence of approximately one in 15,000. It is characterized by severe infantile hypotonia with feeding problems, global developmental delay and mental deficiency, behavior problems, small hands and feet, hypogonadism and hyperphagia leading to marked obesity in early childhood, and a characteristic face.
Read More »POC panel talks diabetes care, data management, CGM
March 2020—Data management, diabetes care, the demand for continuous glucose monitoring, and device cleansing were up for discussion when CAP TODAY publisher Bob McGonnagle led a point-of-care glucose testing roundtable in January. Joining him were Todd Cullen of Arkray, Corinne Fantz, PhD, DABCC, of Roche, and Susan Fuhrman, MD, of OhioHealth Laboratory Services. Here is what they said.
Study: Combined DNA-RNA testing improves detection of MET mutations
March 2020—DNA and RNA sequencing, when used together, can improve detection of MET exon 14 skipping mutations in lung adenocarcinoma compared with DNA testing alone, according to a study reported last November at the Association for Molecular Pathology meeting. While RNA analysis can play an important complementary role to DNA analysis in detecting the mutation, it can also pick up false-positives if RNA analysis data are not adjusted properly, David Manthei, MD, PhD, a fellow in the University of Michigan Department of Pathology, explained in presenting the data. In the study, 482 cases of non-small cell lung cancer were sequenced using the Oncomine Focus Assay, a Thermo Fisher Scientific next-generation sequencing assay that conducts DNA and RNA analysis in a single workflow.
Read More »Put It on the Board
March 2020—Randox has made available a test for the COVID-19 strain of coronavirus. Developed on Randox’s biochip technology, the test is an enhanced multiplex array that includes tests for COVID-19 and nine other respiratory viruses that can manifest the same symptoms. Read more.
Read More »Q&A column
Q. Is there a place for procalcitonin testing alongside PCR testing on the BioFire system? Read answer.
Q. We are looking into validation methods for lower breakpoints for carbapenems. The CDC has banks of organisms available to run on our Vitek 2 (BioMerieux). Are there guidelines on which organisms to include and how many times to test? Read answer.
From the President’s Desk: The federal government and your compensation
March 2020—It is important to understand that we—those of us who work in the U.S.—are all essentially independent contractors for the federal government. Pathologists who work directly for a federal agency or the Department of Defense are the only exception. The federal government has enormous influence on funding and reimbursement in health care. Including direct and indirect spending, about 50 cents of each health care dollar comes from Washington.
Read More »Newsbytes
March 2020—At trauma hospitals, simplicity is considered a virtue. That’s why when Jansen Seheult, MD, and his colleagues decided to use machine learning to predict massive transfusion needs, they chose a decision tree algorithm. “It was easy to implement as if/then rules, and it didn’t require computational resources to deploy,” says Dr. Seheult, clinical assistant professor of pathology at the University of Pittsburgh Medical Center.
Read More »Shorts on Standards: Estimating measurement uncertainty
March 2020—The CAP has 30 official liaisons to various organizations who attend scientific meetings or designate others to do so. They report to the Standards Committee, which reports to the Council on Scientific Affairs. We periodically publish bits of what the CAP’s outbound liaisons hear and see in their liaison roles.
Read More »Clinical Pathology Selected Abstracts
March 2020—U.S. guidelines for human papillomavirus vaccination are 11 to 12 years, with a catch-up vaccination up to age 26 for women and 21 for men. The FDA recently expanded the approved age for HPV vaccination in adult women and men from nine through 45 years. The changes are based on safety data and efficacy as well as potential incremental population-level health benefits. The authors conducted a study to evaluate the added population-level effectiveness and cost-effectiveness of extending the current U.S. HPV vaccination program to women ages 27 through 45 years and men ages 22 through 45 years. They used HPV-ADVISE (agent-based dynamic model for vaccination and screening evaluation), a model for HPV infection and associated diseases specific to U.S. data.
Read More »Molecular Pathology Selected Abstracts
March 2020—Microsatellite instability status in solid tumors is a critical biomarker for predicting tumor response to an immune checkpoint inhibitor drug. The immune system is more likely to attack those tumors that have a high degree of microsatellite instability, a consequence of the genomic instability that also leads to generation of the neoantigens the immune system is designed to recognize.
Read More »Anatomic Pathology Abstracts
March 2020—Conflicting data about the clinical significance of microscopic Crohn disease activity at resection margins have led to varying practice patterns for routine reporting by pathologists. The authors performed a multicenter retrospective cohort study of 101 consecutive Crohn disease bowel resections during a 10-year period to characterize the association between active disease at resection margins and postoperative Crohn disease recurrence and time to recurrence. Margin slides were reviewed, and Crohn disease activity at the margins was graded as none, mild, moderate, or severe. The authors used logistic regression and Cox regression analyses, respectively, to evaluate the association between microscopic Crohn disease activity at the margins and postoperative recurrence and time to recurrence.
Read More »PGx testing wave runs uphill and down
February 2020—Human endeavors are bursting with unintended consequences. Kudzu comes to mind. Smokestacks. Some even point fingers at Smokey Bear. John Greden, MD, offers an example of his own, one with renewed relevance in pharmacogenomics. It’s a subject he’s studied closely, including as principal investigator of the GUIDED trial Researchers looked at whether offering clinicians access to a pharmacogenomics test report would improve treatment for more than 1,100 patients with a major depressive disorder who had already failed to respond to an average of 3.5 antidepressant trials.
Read More »Biomarker screen makes case for MODY genetic testing
February 2020—Cost-effectiveness analysis of health care diagnosis and treatment, unfortunately connoting quotas and spartan budgets, may not have the best reputation among the general public.
Read More »A walk-through on 2 curbside consults
February 2020—In vivo hemolysis accounts for less than two percent of cases of hemolysis, but don’t assume all hemolysis is in vitro. That was the point of one of the patient cases presented at CAP19 in a session on curbside consults in clinical pathology.
Read More »Technological advances enhance liquid biopsy’s clout
February 2020—New microfluidic and nanotechnologies could take liquid biopsy to the next level as a tool to gauge cancer progression and treatment response.
Read More »In memoriam
February 2020—Herbert Derman, MD, CAP president from 1983 to 1985, died Dec. 18, 2019, seeking a remedy to his chronic heart failure. Dr. Derman was CAP vice president from 1981 to 1983 and a member of the Board of Governors from 1971 to 1980. He was the chair and a member of several CAP councils, committees, and commissions. Dr. Derman was named CAP Pathologist of the Year in 1986. He received the ASCP/CAP Pathology Continuing Medical Education award in 1974 and 1978 and the ASCP/CAP Joint Distinguished Service award in 1993.
Read More »Can machine learning algorithms predict lab values?
February 2020—At Massachusetts General Hospital, machine learning is being used in the laboratories to build next-level clinical decision support, and in the latest phase, it’s undergoing trial for use in predicting laboratory results. “I think this is the new paradigm for cost-effective laboratory medicine. This is an important way we’re going to change how we do business,” says Anand Dighe, MD, PhD, who spoke about machine learning techniques for labs during a CAP19 presentation last fall and in a recent interview with CAP TODAY.
Read More »AMP case report: Frameshift and in-frame CALR exon 9 genetic alterations detected in a post-ET myelofibrosis patient before and after stem cell transplantation
February 2020—Myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell disorders characterized by the proliferation of one or more of the myeloid lineages. Philadelphia chromosome-negative classical MPNs include polycythemia vera (PV), primary myelofibrosis (PMF), and essential thrombocythemia (ET).
Read More »Using microfluidics to isolate circulating leukemia cells
February 2020—Microfluidic assays are being used to isolate circulating leukemia cells and manage minimal residual disease in some patients with acute myeloid leukemia and B-cell/T-cell acute lymphoblastic leukemia. “There is a lot of popularity in liquid biopsies, but there’s still a lot of work to do,” said Steven A. Soper, PhD, foundation distinguished professor of chemistry, mechanical engineering, and bioengineering at the University of Kansas. Dr. Soper, who also holds a teaching appointment at Ulsan National Institute of Science and Technology in Ulsan, South Korea, was a co-presenter with Sunitha Nagrath, PhD (see story, page 3), at the 2019 AMP annual meeting.
Read More »AP LIS panel: complexity, middleware, reports, AI
February 2020—Middleware, transmitting and consuming reports, and artificial intelligence are just some of what AP LIS roundtable panelists talked about in December with CAP TODAY publisher Bob McGonnagle. Members of the panel were pathologists Monica de Baca, MD, and Jeffrey Prichard, DO, Rick Callahan of NovoPath, David Liberman, MD, of Computer Trust, and Chad Meyers of Sunquest. Here is what they said.
Read More »Put It on the Board
Roche, Illumina partner to broaden access to genomic testing
February 2020—Roche has entered into a 15-year, nonexclusive partnership with Illumina to broaden the adoption of distributable next-generation-sequencing–based testing in oncology. The agreement brings together the capabilities of both companies to broaden adoption of NGS in cancer care. As part of the agreement, Illumina will grant Roche rights to develop and distribute in vitro diagnostic tests on Illumina’s NextSeq 550Dx system and on its future portfolio of diagnostic sequencing systems. Roche will in turn collaborate with Illumina to complement Illumina’s comprehensive pan-cancer assay TruSight Oncology 500 with new companion diagnostic claims. The financial terms of the deal were not disclosed. Under the IVD terms of the agreement, Roche will develop, manufacture, and commercialize Avenio IVD tests for tissue and blood for use on Illumina’s NextSeq 550Dx. Illumina will continue to sell the NextSeq 550Dx systems and core sequencing consumables.
Q&A column
Q. Does the CAP have recommendations regarding diagnostic criteria for evaluating HER2 in colorectal carcinoma? Read answer.
Read More »From the President’s Desk: Our first Leadership Summit
February 2020—Regular readers of CAP TODAY know I believe that it is extremely important for CAP members to become politically active. The upcoming Pathologists Leadership Summit, which will take place May 2–5 in Washington, DC, will give you the opportunity to do so. If you haven’t registered already, I encourage you to consider attending this special event. The Pathologists Leadership Summit is new for the CAP. It is replacing our annual policy meeting focused on government advocacy.
Read More »Newsbytes
Drone delivery of lab samples: from progress at WakeMed to interest elsewhere
February 2020—After nearly a year of a drone buzzing through the air to deliver specimens from the Raleigh Medical Park surgery center to the laboratory at the flagship Raleigh campus of WakeMed Health and Hospitals a quarter-mile away, WakeMed is looking to expand its drone program. Read More »Clinical Pathology Selected Abstracts
Blood utilization and transfusion reactions in pediatric patients transfused with platelets
February 2020—Even with advances in donor screening and infectious disease testing, the risk of transfusion-transmitted infections continues to be a concern. The FDA has approved a pathogen-reduction system for single-donor platelets, called Intercept Blood System (Cerus Corp.), to treat thrombocytopenic adult and pediatric patients. Read More »Anatomic Pathology Selected Abstracts
DNAJB1-PRKACA fusions in fibrolamellar hepatocellular carcinoma
February 2020—Recently discovered DNAJB1-PRKACA oncogenic fusions have been considered diagnostic for fibrolamellar hepatocellular carcinoma. The authors conducted a study in which they described six pancreatobiliary neoplasms with PRKACA fusions, five of which harbored the DNAJB1-PRKACA fusion. Read More »Molecular Pathology Selected Abstracts
Overall survival rates for lung cancer patients receiving osimertinib
Guidelines recommend testing patients with advanced or metastatic nonsmall cell lung cancer (NSCLC) for epidermal growth factor receptor (EGFR) alterations because targeted treatment with a tyrosine kinase inhibitor (TKI) is available for patients with EGFR exon 19 deletions or L858R point mutations. Osimertinib is a third-generation, irreversible, oral EGFR-TKI that selectively inhibits EGFR-TKI–sensitizing and EGFR p.Thr790Met-resistance mutations. Read More »D-dimer trifecta: clarity on units, values, and use
January 2020—D-dimer has a problem. Several problems, in fact. Many, some might say. Let’s start with the basics regarding D-dimer assays: unit and magnitude. Setting up the equation is easy, and the digits are small: 2 × 4. When applied to D-dimer testing, however, the answer often means far-flung problems for laboratories and clinicians. Parsed out (for fans of James Thurber, this means channeling schoolmarm Miss Groby), D-dimer has two different units of measure. Some assays report fibrinogen equivalent units (FEU), and others measure D-dimer units (DDU).
Read More »No-blot testing charts new course for Lyme Dx
January 2020—“You can eliminate blots altogether!” may sound like a pitch for a cleaning product. But it’s a line from a webpage of Zeus Scientific touting the new algorithm for Lyme disease testing that ditches Western immunoblot in favor of a two-enzyme immunoassay test sequence. The new algorithm, which the CDC recommended last year, is the first approved alternative to the standard algorithm in 25 years.
Read More »Carcinoma of unknown primary case reviewed in tumor board session
January 2020—A molecular oncology tumor board session at CAP19 explored a case of cancer of unknown primary, presented by medical oncologist Alexander Drilon, MD, research director of early drug development at Memorial Sloan Kettering Cancer Center, and Rondell P. Graham, MBBS, head of GI/liver pathology at Mayo Clinic Rochester.
Read More »Study: Subpar reporting of biomarker characteristics
January 2020—Since the Journal of Irreproducible Results arrived on the scene in 1955, founded by a physicist and a virologist, its parodies of scientific research have amused and skewered many in the scientific community. But in the world of clinical research, irreproducibility is a less than whimsical idea.
Read More »At NCI, on the trail of drug-induced liver injury
January 2020—When looking at a liver biopsy, always suspect there might be drug-induced liver injury. “But then try hard to prove there isn’t. It’s always a diagnosis of exclusion and pattern evaluation,” says David E. Kleiner, MD, PhD, reference pathologist for the Drug-Induced Liver Injury Network. In that role, he pieces together clues that point to a drug having injured a liver—or not. Drug-induced liver injury is rare, and few pathologists see it. “The incidence is between one in 100,000 and one in a million, depending on what survey you read,” Dr. Kleiner says.
Read More »New edition of toxicology testing guide now out
January 2020—CAP Publications released this month the second edition of Clinical Toxicology Testing—A Guide for Laboratory Professionals, edited by Barbarajean Magnani, PhD, MD; Tai C. Kwong, PhD; Gwendolyn A. McMillin, PhD; and Alan H.B. Wu, PhD. The first edition was published in 2012. The book has 29 chapters divided into three sections: toxicology testing in the clinical setting, toxicokinetics and methodologies for the toxicology laboratory, and specific analytes (drugs and drug classes). CAP TODAY spoke with Dr. Magnani about the new book. She is director of toxicology and chief of clinical pathology, Department of Pathology and Laboratory Medicine, Tufts Medical Center, and professor of anatomic and clinical pathology, and professor of medicine, Tufts University School of Medicine, Boston. Here is what she told us. (See excerpt below.)
Read More »Cytopathology in focus: Non-small cell lung carcinoma
January 2020—Requests for predictive biomarkers in oncology patients are becoming increasingly common in the cytology laboratory. At the time of rapid on-site evaluation, cytologists are now keenly aware of the need to collect adequate material not just for a diagnosis of malignancy but also for diagnostic and predictive molecular and immunohistochemical testing. This article provides an overview of current practices and some of the recent literature regarding predictive testing for immunotherapy in cytologic preparations in non-small cell lung carcinoma.
Read More »Cytopathology in focus: Self-collected Pap tests in the U.S. market
January 2020—The authors of an article published in the Journal of the American Society of Cytopathology participated in a public hearing at the Food and Drug Administration in January 2018. The hearing advised the FDA about what research would be required to demonstrate the safety and efficacy of a self-collected Papanicolaou test device. In the article, Staats, et al., review the literature on self-collected Pap tests. The authors also provide a review of published studies on self-collected HPV tests. They pose important questions that surround the self-collected Pap test; most remain only partially answered, given the limited evidence examining such tests in the literature.
Read More »Cytopathology in focus: Review of FDA-approved molecular testing platforms for HPV
January 2020—The Food and Drug Administration approved in 2001 the first testing modality for the detection of HPV in gynecological cytological specimens. To date, there are now five FDA-approved testing modalities, and molecular testing for high-risk HPV has become commonplace. Numerous studies have shown that high-risk HPV testing is more sensitive in detecting high-grade squamous intraepithelial lesion/cervical intraepithelial neoplasia grade two and above (HSIL/CIN2+) than cytology alone, but that cytology is more specific.
Read More »Put It on the Board
Ion Torrent Genexus launched at AMP meeting
January 2020—Thermo Fisher Scientific launched at the Association for Molecular Pathology meeting in November its Ion Torrent Genexus System. It is a fully integrated, next-generation sequencing platform that features an automated specimen-to-report workflow, with results provided in a single day. The company also introduced its Oncomine Precision Assay, a pan-cancer panel for the Genexus platform, for comprehensive genomic profiling from formalin-fixed, paraffin-embedded tissue and liquid biopsy samples with a single assay. The Genexus System minimizes user intervention and the potential for human error. Thermo Fisher says the system requires minimal amounts of tissue sample and can run small batches cost-effectively to deliver a comprehensive report in one day. Together, the company said in a statement, “these features set the stage for molecular pathologists in the future to analyze NGS information in parallel with first-line testing modalities such as immunohistochemistry.”
Q&A column
Q. How should automated body fluid cell counts be reported? Read answer. Q. Can the CAP provide guidance on revised checklist requirements GEN.77500 Liquid Nitrogen and Dry Ice and GEN.77550 Liquid Nitrogen Safety? Read answer.
Read More »From the President’s Desk: What’s the state of your state pathology society?
January 2020—Recently I attended and spoke at a meeting of the Georgia Association of Pathology. That might not sound like a big deal, but it was. This was an important occasion for me and my colleagues in Georgia because our state society had been dormant for the past decade. Thanks to the efforts of five CAP fellows and the CAP itself, we are back in Georgia.
Read More »Newsbytes
January 2020—Pathologist Ron B. Schifman, MD, practices what he preaches and preaches about what others practice relative to implementing such computer-based test-utilization management techniques as soft stops, hard stops, and those that fall in between. In a 2019 American Association of Clinical Chemistry presentation on strategies and tactics for test-utilization management, and in an interview with CAP TODAY, Dr. Schifman offered insights into a variety of information technology-based interventions.
Read More »Clinical pathology selected abstracts
Use of thromboelastography to guide blood product transfusion
January 2020—Thromboelastography and rotational thromboelastometry provide insights into blood clot development, stabilization, and dissolution. The coagulation tests provide a tracing through the clotting process, but although they are similar, they are not interchangeable.
Anatomic pathology selected abstracts
Distinct patterns of human liver regeneration following massive hepatic necrosis
January 2020—Massive hepatic necrosis is a rare and often fatal complication of various liver injuries. However, some patients survive by spontaneous hepatic regeneration. It is known that surviving hepatocytes or progenitor cells, or both, can participate in this process, but the mechanism of hepatic recovery is vague.
Molecular pathology selected abstracts
Virtual staining of tissue slides to conserve precious diagnostic samples
January 2020—Precise classification of neoplasms improves risk stratification and the ability to apply targeted treatment options, enhancing patient care. These granular diagnostic classifications increasingly rely on molecular findings that go beyond what the microscope shows the pathologist.
OncoLens offers free virtual tumor board platform
In response to the ongoing challenges for cancer care teams due to the COVID-19 pandemic, OncoLens is offering its OncoLens virtual tumor board software at no cost for 60 days after signing a business associate agreement, through May 15, 2020. The module enables cancer care teams to meet virtually, in real time, to discuss treatment options for their cancer patients on a secure, HIPAA-compliant platform.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management