August 2020—In March, the COVID-19 pandemic came in like a lion—and has yet to leave, like a lamb or anything else. Instead, it roared through April and May in early hot spots like New York City and New Orleans. As lockdowns took hold, the cautious hope was that by summer the virus would be tamed (if not simply go away “like a miracle” or “as the heat comes in,” per several infamous predictions), giving health care providers a chance to exhale before a likely second wave in the fall. Instead, June and July saw other cities and states hit hard in turn, while many places that appeared to have flattened the curve were starting to see concerning upticks in cases.
Read More »August 2020
Targeting microbiology lab efficiency with AI
August 2020—Bringing an automated culture plate reading system into the Hennepin County Medical Center microbiology laboratory was never a question of if but when. “We need artificial intelligence to help us with active decision-making processes in the lab,” says Glen Hansen, PhD.
Read More »In 2020 checklist, a ‘gentle push’ to next quality level
August 2020—For quality management in the laboratory, it’s not enough to have checks and balances. The checks and balances have to work to improve quality. That’s how Stephen J. Sarewitz, MD, vice chair of the CAP Checklists Committee, characterizes the changes to the quality management requirements in the 2020 laboratory general checklist, released in June.
Read More »Lab with Ebola experience: COVID more complicated
August 2020—If there’s one thing scarier to experience than COVID-19, it’s Ebola. Or so you might think. “Ebola was easier,” says Beverly Dickson, MD, medical director of the clinical laboratory at Texas Health Presbyterian Hospital Dallas.
Read More »Steps to verifying SARS-CoV-2 antibody assays and what’s known about protective immunity
August 2020—The CAP treats emergency use authorization assays similar to FDA-cleared assays and thus requires full verification. In a June 4 CAP webinar, Neil Anderson, MD, D(ABMM), assistant director of clinical microbiology, Washington University School of Medicine in St. Louis, walked through how to approach verification for SARS-CoV-2 assays. Co-presenter Elitza Theel, PhD, D(ABMM), director of the infectious diseases serology laboratory at Mayo Clinic, reported what’s known about protective immunity against SARS-CoV-2.
Read More »Tumor budding assessment in CRC: why and how
August 2020—Tumor budding is a robust prognostic marker that should be reported at least in pT1 and stage II colorectal carcinomas and taken into account with other risk factors. Further evidence is needed for tumor budding assessment in specimens taken after neoadjuvant therapy, says Heather Dawson, MD, senior staff GI pathologist at the Institute of Pathology, University of Bern in Switzerland.
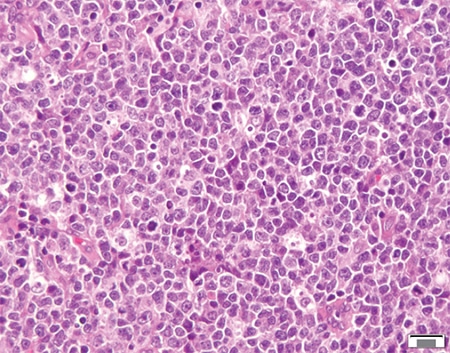
Read More »AMP case report: Burkitt-like lymphoma with 11q aberration
August 2020—Burkitt-like lymphoma with 11q aberration (BLL-11q) is a new provisional entity in the revised 2016 WHO classification of hematopoietic and lymphoid tumors.1 It refers to a subset of high-grade B-cell lymphomas that resemble Burkitt lymphoma with similar morphology, phenotype, and gene expression profiling, but lack MYC gene rearrangements. Instead, these lymphomas carry chromosome 11q proximal gains and telomeric losses, suggesting co-dysregulation of oncogenes and tumor suppressor genes.
Read More »COVID testing capacity falls short as flu season nears
August 2020—As the need for COVID-19 testing grows well beyond that for hospital patients, clinical laboratories in mid-summer were again overwhelmed by demand while at the same time bracing for flu season. That was the gist of a July 10 webinar that brought together Gyorgy Abel, MD, PhD, medical director of clinical chemistry, molecular diagnostics, immunology, and point-of-care testing at Lahey Hospital and Medical Center, Burlington, Mass.; Bob McGonnagle, CAP TODAY publisher; and moderator Steve Beuchaw, director of life science and medical device research, Wolfe Research.
Read More »Cytopathology in focus: Abnormal cervical screening tests: a personal approach
August 2020—If the past decade was directed toward aligning medicine with a personalized approach to therapy, this decade should further realize the implementation of health care decisions tailored to the patient. The updated 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors take a large step in that direction by relying on the input of personal data into a free online application that provides suggested management planning based on patient history and prior Pap/HPV test results.
Read More »Cytopathology in focus: New direction for thoracic small biopsy, cytology specimens
August 2020—Cytopathologists are keenly aware of the need to collect adequate cytologic tissue not only to arrive at a diagnosis but also to provide sufficient material for predictive and prognostic markers. This is especially true in the realm of non-small cell lung cancer, where biomarker testing is routinely used for the clinical management of patients with advanced-stage disease. The list of clinically relevant biomarkers in NSCLC is expanding. The most recent version of the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology includes MET exon 14 skipping mutations and RET as therapeutic targets for advanced NSCLC, in addition to the well-established EGFR and BRAF mutations, ALK and ROS1 rearrangements, and PD-L1 expression.
Read More »From the President’s Desk: Virtual meetings, for now
August 2020—In the past six months, many of us have developed video conferencing skills to an extent we never imagined we would need. Until the COVID-19 pandemic led to lockdowns in several states, a great deal of expertise in Zoom and other similar platforms was not a high priority among many pathologists. I am proud that our community adapted quickly and skillfully to continue providing the best care possible to our patients.
Read More »Clinical pathology selected abstracts
August 2020—Bacterial contamination of platelets continues to be an important cause of transfusion-associated morbidity and mortality. From 2001 to 2016, there were 51 deaths reported to the FDA due to transfusion of apheresis products contaminated with bacteria, including 30 deaths since testing for bacterial contamination was mandated in 2004. This mandate was implemented through primary culture of single-donor apheresis platelets in 2004 and then prestorage pooled platelets (PSPPs) in 2007. The authors conducted a study to compare the platelet bacterial contamination and septic transfusion rates before and after introducing testing of pooled and apheresis platelets by primary culture over an extended time period. They cultured platelet aliquots at issue and evaluated transfusion reactions.
Read More »Anatomic pathology selected abstracts
August 2020—The authors conducted a study in which they reviewed 354 cases of malignant diffuse mesothelioma in women from a database of 2,858 histologically confirmed cases. Pleural predominance was noted with 78 percent of pleural malignant mesotheliomas (MM) and 22 percent of peritoneal MM. The pleural tumors consisted of 72 percent epithelioid, 19 percent biphasic, and nine percent sarcomatoid variant. The peritoneal tumors consisted of 82 percent epithelioid, 13 percent biphasic, and five percent sarcomatoid. The immunohistochemical profile was typical of what is well accepted and previously described for MM.
Read More »Molecular pathology selected abstracts
August 2020—Patients and families suspected of having Mendelian diseases, syndromes thought to be caused by a single mutated gene, often undergo comprehensive next-generation sequencing to determine the underlying pathogenic variant. Whole exome sequencing, in which the coding regions of all genes are analyzed, is usually the preferred testing method. However, this identifies the diagnostic pathogenic variant in only 25 to 52 percent of cases. Whole genome sequencing appears to confer only marginal benefit over whole exome sequencing, presumably because the pathogenic variants likely are not missed by whole exome sequencing but may be misinterpreted as nondiagnostic. Among the variants that are difficult to interpret are those that affect RNA by directly influencing transcription, or altering normal splicing, or by mediating their effects through chromatin.
Read More »Q&A column
Q. If a person died from an overdose, would the toxicology screen always show which drugs were in their system? Read answer. Q. We are looking into using a scale to measure 24-hour urine samples, but we can’t find much literature about it. Is the variation between the measurement from a scale and actual volume clinically significant? What kind of validation is recommended? Read answer.
Read More »Newsbytes
August 2020—At Sonora Quest Laboratories, working backward has been a key strategy for leaping forward. Little by little, the Arizona-based integrated laboratory system has been retracing paper trails and assessing established processes as part of an ambitious plan to eliminate paper use across its seven commercial laboratories, 28 hospital labs, and more than 75 patient service centers.
Read More »Put It on the Board
August 2020—SpIntellx and CellNetix Pathology & Laboratories will collaborate to validate the SpIntellx HistoMapr-Breast Platform. The platform is the first in the companies’ larger efforts to use explainable artificial intelligence-assisted lab processes in the development of diagnostics, prognostics, therapeutic strategies, and drug development for tumor and non-tumor diseases.
Read More »Published in June:
At the pandemic’s serologic frontier
 The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves. That also meant months, possibly stretching into summer, in which laboratories faced more questions than answers, at a time when the demand for serology tests seems insatiable (and somewhat perplexing).
The arrival of a pandemic has shown—among many, many other things—that anyone who talks about it typically starts by saying, “This is a pandemic.” The next sentence tends to be, “It’s a completely different situation,” whether the focus is grocery shopping, exercising (or not), voting, or practicing medicine. Pointing to the pandemic is a polite way of saying, “All bets are off.” For many, it’s been a springboard to innovation and breakthroughs, even in the midst of considerable anguish. For clinical laboratories, however, much has felt unsettling, especially when the conversation turns to serology testing for SARS-CoV-2. It’s a topic stuffed to overflowing with interest, enthusiasm—and, early on, antibody tests themselves. That also meant months, possibly stretching into summer, in which laboratories faced more questions than answers, at a time when the demand for serology tests seems insatiable (and somewhat perplexing).
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management