CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of New Mexico. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Audra Kerwin, MD
Devon Chabot-Richards, MD
Laura Toth, DO, MPH
April 2021—A 71-year-old female with a history of asthma and hypertension initially presented to her local hospital complaining of shortness of breath. She was found to be pancytopenic with severe anemia (hemoglobin 5 g/dL). She was subsequently transferred to a tertiary care facility for further evaluation.
Bone marrow biopsy revealed a hypercellular marrow composed of 72 percent blasts (Fig. 1). Flow cytometric analysis revealed a B-lymphoblast immunophenotype with expression of CD34, dim CD45, CD19, CD79a, CD22, HLA-DR, TDT, CD200, CD33, and dim CD13. The blasts were negative for MPO, CD3, CD4, CD7, CD8, CD10, CD20, CD14, CD56, CD64, and CD117. Cytogenetic analysis identified a complex female karyotype exhibiting extensive numerical and structural abnormalities, including interstitial deletion of the long arm of chromosome five and an additional copy of BCR (22q11.2) (Fig. 2).
As these findings did not fulfill the diagnostic criteria for a subcategory of B-lymphoblastic leukemia/lymphoma (B-ALL) with recurrent genetic abnormality, screening for a BCR-ABL1-like RNA expression profile was performed using a low-density array card (commercially available from TriCore Reference Laboratories). This was positive for a BCR-ABL1-like profile with low CRLF2 expression (Fig. 3), consistent with BCR-ABL1-like B-ALL not due to CRLF2 rearrangement. Reflex FISH was negative for rearrangements involving CRLF2, ABL1, ABL2, CSF1R, EPOR, and PDGFRB. Further DNA and RNA sequencing was considered to identify the specific genetic alteration causing the BCR-ABL1-like profile. However, the oncologist decided to proceed with treatment for acute lymphoblastic leukemia, BCR-ABL1-like.
Given the diagnosis of BCR-ABL1-like B-ALL, the patient was treated with an aggressive chemotherapeutic regimen that included the addition of inotuzumab and intrathecal methotrexate. After induction, repeat bone marrow showed the presence of residual disease with a blast count of 8.3 percent. The patient eventually achieved morphologic remission after three months of intensive chemotherapy. Seven months later she returned with symptoms including fatigue and shortness of breath. Bone marrow biopsy at that time demonstrated dysplastic features and increased blasts of myeloid lineage (CD34, CD33, CD13, CD117, and subset MPO positive by flow cytometric analysis, negative for CD19, CD20, and TdT), and a diagnosis of therapy-related myeloid neoplasm (t-MN) was rendered (Fig. 4).
Cytogenetic analysis revealed a karyotype with shared abnormalities to that of the patient’s original BCR-ABL1-like B-ALL, including the interstitial deletion of the long arm of chromosome five (Fig. 5). This may indicate a clonal relationship between the two, including underlying clonal hematopoiesis or the rare phenomenon of lineage switching. In this event, leukemic cells immunophenotypically and morphologically convert to a different lineage. The exact biological mechanism underlying lineage switch remains elusive, but molecular and cytogenetic analysis of these events has uncovered interesting findings that may indicate therapy-related clonal selection.1 However, this rare yet well-described event typically occurs in a short time frame, immediately after or even during induction therapy. Thus, the diagnosis of t-MN was rendered.1 The patient died three months later.
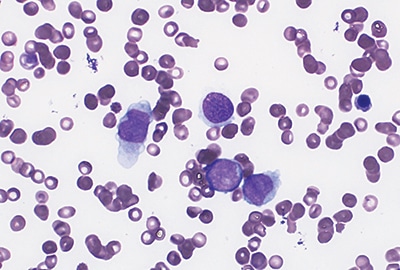
Fig. 1. Bone marrow aspirate at diagnosis of ALL shows blasts with large size, high nuclear-to-cytoplasmic ratio, and prominent nucleoli (Wright stain, 40×).
B-lymphoblastic leukemia/lymphoma, BCR-ABL1-like. The category of B-lymphoblastic leukemia/lymphoma (B-ALL) with recurrent genetic abnormalities has expanded over time and is now composed of nine distinct entities in the latest revision of the World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. The update includes two new diagnostic subcategories: B-ALL with intrachromosomal amplification of chromosome 21 and B-ALL, BCR-ABL1-like.2 BCR-ABL1-like ALL is a genetically heterogeneous group of diseases showing gene expression profiles similar to B-ALL with the BCR-ABL1 translocation. These leukemias often carry translocations of other tyrosine kinase genes as well as CRLF2 and EPOR. Seen in 10 to 20 percent of pediatric ALL, the incidence of BCR-ABL1-like increases with age, reaching 20 to 30 percent in adult ALL. Although originally described in pediatric patients, identification is of particular importance in adults because it portends a significantly worse overall and event-free survival, with a five-year survival of approximately 23 percent.3,4
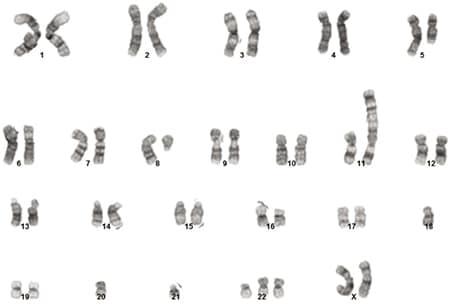
Fig. 2. Karyogram at the initial time of diagnosis with a composite karyotype of 46,XX,del(5)(q22q35)[2]/44-46,idem,del(8)(q11.2),add(11)(p15),del(16)(p12),
-18,-20,-21,+22,add(22)(p11.1),add(22)(p11.1),+mar[cp17]/46,XX[1].
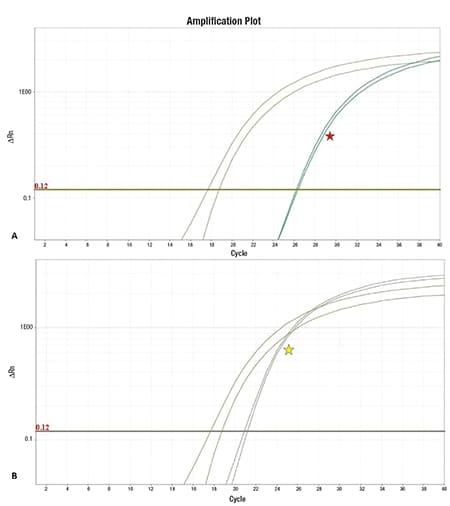
Fig. 3. The determination of a Ph-like profile is made via a low-density array, which calculates a composite RNA expression score for a set of 15 genes based on the PCR cycle when signal is detected compared with a control gene (olive green). A strict numerical cutoff for determining increased expression of each individual gene cannot be provided because each gene is internally normalized for each specific case (further description can be found in the supplemental information of reference 10). A) One gene evaluated is CRLF2, which was low as shown above (red star). B) A second gene evaluated, IGJ, shows relatively increased expression (yellow star).
The most commonly reported abnormality is rearrangement of the CRLF2 gene leading to increased expression (30 to 50 percent of cases).6 Common translocation partners include IgH and P2RY8.7 Additionally, 30 to 50 percent of these CRLF2-rearranged cases harbor a concurrent JAK1 or JAK2 activating mutation that may be amenable to targeted therapy with agents such as ruxolitinib in combination with conventional chemotherapy.6 Additional targetable rearrangements associated with a BCR-ABL1-like profile include EPOR and the ABL class of genes: ABL1, ABL2, CSF1R, PDGFRA, and PDGFRB, which may be responsive to the addition of dasatinib or imatinib.3,4,7,8 Many of these rearrangements can be evaluated using FISH. Additional fusions and mutations leading to increased kinase signaling, including IKZF1, FGFR1, and RAS, have been less commonly reported.4
Based on current guidelines, patients who should undergo BCR-ABL1-like screening should include all children diagnosed with B-ALL who fall into the high-risk category, other than those with the t(9;22)(q34.1;q11.2) BCR-ABL1 and t(12;21)(p13.2;q22.1) ETV6-RUNX1, which exclude the diagnosis BCR-ABL1-like B-ALL. Children with standard-risk B-ALL may be considered for screening if there is residual disease after induction, or if there is central nervous system or testicular involvement.6 Finally, as in this case, adults with B-ALL who are negative for the t(9;22)(q34.1;q11.2), BCR-ABL1 gene fusion should also be screened.6
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
