CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of California, San Francisco. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Kiavash Garakani, MS
Patrick Devine, MD, PhD
Roberto Ruiz-Cordero, MDM
May 2020—Traditionally, histopathologic diagnosis has been regarded as the gold standard for most disease processes including cancer. However, in certain circumstances, a final histopathologic diagnosis cannot be rendered despite extensive conventional ancillary testing such as immunohistochemistry. In recent years, molecular testing has revealed specific variant signatures for many tumors, which can be used to determine a final diagnosis. Herein, we present two such cases of histologically complex tumors with indefinite immunophenotype whose differential diagnoses after extensive histopathologic workup included several tumor entities with very different treatments and prognoses. We assessed the variant profile of both tumors using a capture-based comprehensive next-generation sequencing panel performed at the UCSF clinical cancer genomics laboratory on an assay (UCSF500 panel) that targets the coding regions of 480 cancer-related genes, select introns from approximately 40 genes, and the TERT promoter with a total sequencing footprint of 2.8 Mb. Sequencing was performed using formalin-fixed, paraffin-embedded tissue sections and matched normal blood on an Illumina HiSeq 2500 sequencer.
Case report No. 1. The first patient is a 52-year-old perimenopausal woman with a past medical history of uterine smooth muscle tumor of uncertain malignant potential (STUMP) status post supracervical hysterectomy, bilateral salpingectomy, and lysis of adhesions who presented to urgent care with a sudden onset of severe left lower quadrant pain that later became generalized. A CT scan revealed a new pelvic mass, measuring 11.6 × 8.9 × 11.4 cm with predominantly solid and minor cystic components in the midline pelvis. An MRI was also ordered and confirmed the mass, with concern for bladder invasion and nodal disease of right presacral nodes. The patient underwent exploratory laparotomy, radical trachelectomy, bilateral oophorectomy, pelvic node biopsy, infracolic omental biopsy, rectosigmoid resection with end-to-end reanastomosis, cystoscopy with bilateral ureteral stent placement, and appendectomy.
On histological examination, the pelvic tumor invaded the colonic serosa, with nodules in the pericolonic adipose tissue (Fig. 1A and B) and the serosa and stroma of the upper endocervix with negative margins. No lymph nodes were positive. The tumor sections showed fascicles (Fig. 1C, upper) and nests of ovoid to spindle neoplastic cells with moderate cytological atypia, brisk mitotic figures (10/10 HPFs) (Fig. 1C, mid), and no necrosis but areas with myxoid changes (Fig. 1C, bottom). The differential diagnosis included leiomyosarcoma, malignant perivascular epithelioid cell neoplasm (PEComa), inflammatory myofibroblastic tumor, endometrial stromal sarcoma, and gastrointestinal stromal tumor (GIST). Immunohistochemical staining showed positivity for desmin, ER (strong, diffuse), DOG1 (patchy), scattered positivity for HMB45 (Fig. 1D), MITF (scattered), fumarate hydratase (retained), SMA, and caldesmon and negativity for CD117, STAT6, melan A, and ALK (not shown). The positivity for smooth muscle markers and focal HMB45 positivity narrowed the differential diagnosis to leiomyosarcoma and PEComa, which is a tumor showing both smooth muscle and melanocytic differentiation. The significance of the patchy positivity of DOG1 is unclear. The patient is currently alive and undergoing chemotherapy for peritoneal disease.
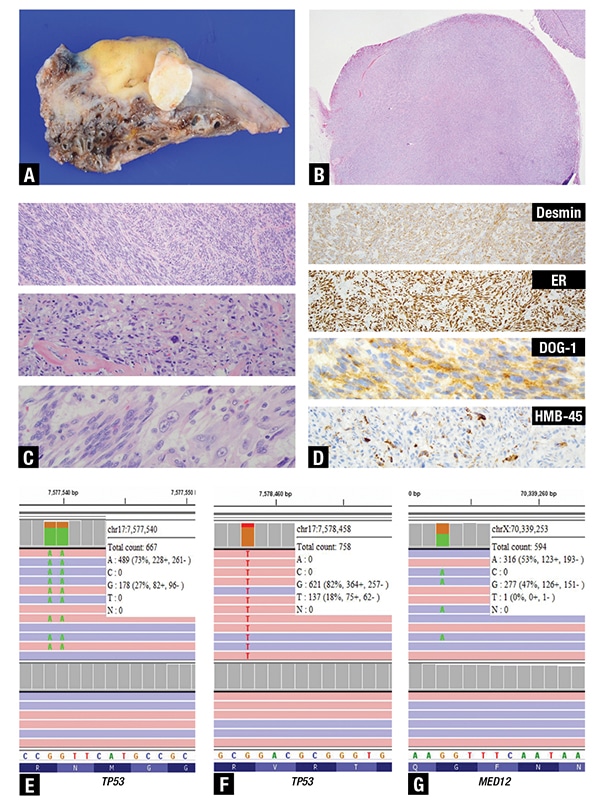
Fig 1. A. Gross image of a portion of the pelvic tumor invading the colonic serosa (yellow-tan area) with a white oval nodule of tumor in the pericolonic adipose tissue. B. Microscopic examination of the white oval nodule shows a well-circumscribed nodule within fat (H&E, 4×). C. Higher magnification of the tumor shows variable histology including fascicles (upper pane, H&E, 10×), groups of ovoid to spindle tumor cells with moderate cytological atypia and mitotic activity in a background of mixed inflammatory cells and collagenous fibrosis (middle pane, H&E, 20×), and areas with myxoid degeneration without necrosis (bottom pane, H&E, 40×). D. By immunohistochemistry, the tumor cells showed diffuse cytoplasmic positivity for desmin (upper pane, IHC, 10×), strong nuclear positivity for estrogen receptor (mid upper pane, IHC, 10×), patchy cytoplasmic positivity for DOG1 (mid lower pane, IHC, 40×), and scattered cytoplasmic positivity in a few cells for HMB45 (lower pane, IHC, 20×). Next-generation sequencing results were confirmed using the Integrative Genomics Viewer (IGV). Screenshots of IGV (E–G) show on the top portion the tumor reads and the germline reads below. The white boxes for each of the figures contain the chromosomic location, coverage, and variant allelic frequency (VAF). E. Shows the p.R248W variant in TP53 with loss of heterozygosity. F. Shows the p.R158S missense variant also in TP53 at a lower VAF, suggestive of a subclonal event. G. Shows the hotspot missense variant p.G44S in MED12.
Next-generation sequencing performed on a section showing about 40 percent tumor revealed two pathogenic missense variants in TP53: a clonal missense variant (NM_000546.5(TP53):c.741_742delCCinsTT p.R248W) with loss of heterozygosity (Fig. 1E), and a subclonal missense variant (NM_000546.5 (TP53):c.472C>A p.R158S) (Fig. 1F). Additionally, a missense variant in MED12 (NM_005120.2(MED12): c.130G>A p.G44S) was identified (Fig. 1G). No variants in TSC1 or TSC2 or rearrangements in TFE3 were detected. The presence of TP53 and MED12 variants, as well as the absence of alterations in the PEComa-associated TSC1, TSC2, and TFE3 genes supported the final diagnosis of leiomyosarcoma.1 Since PEComas may be sensitive to mTOR inhibitor therapy, the right definitive diagnosis between the PEComa and leiomyosarcoma carried significant therapeutic implications.
Case report No. 2. Our second patient was a 72-year-old woman with a few-month history of worsening shortness of breath, morning cough, and difficulty swallowing who was found to have a left thyroid nodule with an associated left upper mediastinal mass encasing the left carotid artery and compressing the trachea (Fig. 2A). Fine-needle aspiration of the thyroid revealed a papillary thyroid carcinoma. She underwent core needle biopsies of the left upper mediastinal mass that were inconclusive; therefore, an open biopsy was performed and was initially interpreted as undifferentiated thymic carcinoma. The histologic material was sent in consultation after the patient presented to our institution for a second opinion. In-house pathology assessment suggested a poorly differentiated carcinoma with a differential between anaplastic thyroid carcinoma and thymic carcinoma.
Histology showed a poorly differentiated malignant neoplasm composed of a few large, loosely cohesive spindled-to-epithelioid cells infiltrating fibroconnective tissue and skeletal muscle within a background of prominent chronic inflammation and fibrosis (Fig. 2B and inset). The tumor showed large areas of necrosis. On morphology, the differential diagnosis included lymphoma, undifferentiated thymic carcinoma, and anaplastic thyroid carcinoma. Immunohistochemical staining showed positivity of the tumor cells for OSCAR cytokeratin (Fig. 2C), patchy positivity for PAX8 (Fig. 2D) and CK7, and negativity for CK20, CK5/6, p40, TTF1, thyroglobulin, and CD5 (not shown). Based on the immunohistochemical staining pattern, an anaplastic thyroid carcinoma was favored, but NGS was suggested for definitive classification. The patient was not a candidate for surgery based on widespread disease (stage IVC) and underwent palliative radiotherapy and chemotherapy. Unfortunately, she experienced progressive hypoxia and weakness and died within a month.
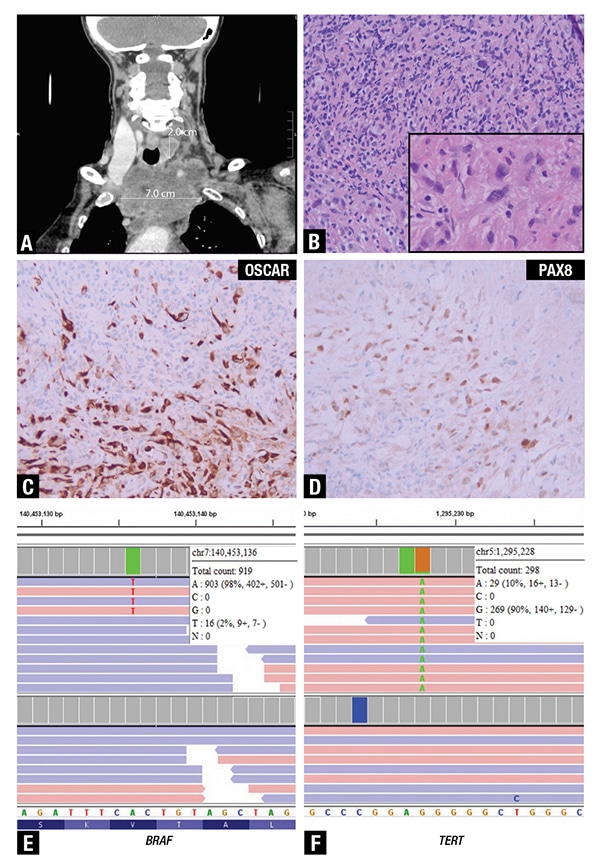
Fig 2. A. Computed tomography in coronal view shows a 2.0-cm nodule in the left thyroid lobe and a 7.0-cm left upper mediastinal mass encasing the left carotid artery and compressing the trachea. B. The histologic sections showed a poorly differentiated malignant neoplasm composed of a few large cells admixed with chronic inflammatory cells (H&E, 20×). The inset highlights a few large, loosely cohesive spindled-to-epithelioid cells within a background of fibrosis and small lymphocytes and plasma cells (H&E, 60×). Immunohistochemical staining showed positivity of the tumor cells for C, OSCAR cytokeratin (IHC, 20×) as well as patchy nuclear positivity for D, PAX8 (IHC, 20×). Screenshots of IGV show E, the hotspot missense variant p.V600E in BRAF at a very low VAF and F, the common pathogenic TERT promoter variant c.-124C>T.
NGS performed on a section showing about 10 percent tumor identified a pathogenic TERT promoter variant (NM_198253.2(TERT): c.-124C>T) at a low variant allelic frequency (VAF) (Fig. 2F). Due to the paucity of the large tumor cells and the prominent lymphocytic and necrotic background, we performed manual review of the hotspot V600E in BRAF and identified a variant (NM_004333(BRAF):c.1799T>A p.V600E) below the lower limit of detection at two percent VAF that was considered a true variant based on the high depth of coverage (919×) and the presence of at least five positive reads in each direction. On the basis of these variants, thymic carcinoma could be excluded, and a diagnosis of anaplastic thyroid carcinoma was established. Distinction between these two entities is important for treatment, particularly in the setting of a BRAF p.V600E mutated tumor that can be treated with targeted therapy.
Discussion. In the first patient, we identified pathogenic MED12 p.G44S and TP53 p.R248W and p.R158S missense variants. Recurrent somatic alterations in the MED12 gene have been found in several tumors, including uterine leiomyosarcomas, uterine and extrauterine leiomyomas, breast fibroadenomas and phyllodes tumors, hematologic malignancies such as chronic lymphoid leukemia, and in rare instances colorectal cancer.2 These variants most often occur in exon 2 at one of three primary hotspot codons—36, 43, or 44—but can also occur less frequently in exon 1 or at other sites within exon 2.3 MED12 is a component of the mediator complex, a large and dynamic multi-subunit protein complex whose core function is to mediate the transcription of all protein-coding and most non-coding RNA. It performs this by serving as a bridge between sequence-specific DNA binding transcription factors and RNA polymerase II.4 Several hotspot variants in MED12, including the MED12 p.G44S variant identified in our leiomyosarcoma patient, have previously been shown to disrupt the interaction of MED12 with the mediator complex subunit cyclin C.3 This leads to the decoupling of cyclin C-CDK8/19 from the core mediator complex, which may promote tumorigenesis through a yet-uncharacterized mechanism.3 MED12 variants occur in about 70 percent of uterine leiomyomas and 15 percent of leiomyosarcomas.5 Somatic TP53 variants are also a relatively common event in leiomyosarcomas, occurring in approximately 24 to 30 percent of cases.5
In the second patient, we identified pathogenic TERT c.-124C>T and BRAF p.V600E variants. TERT promoter variants are a frequent genetic aberration found in several tumors, including melanoma, thyroid cancer, central nervous system tumors, and bladder cancer.6 These variants most commonly occur at hotspot positions -124 (G → A) and -146 (G → A) upstream of the TERT transcription start site,7 and they are thought to increase transcription at the site by generating de novo binding sites for E26 transformation-specific (ETS) transcription factors.6 Since telomerase activity is primarily controlled in cells through the regulation of TERT gene transcription, this increase in transcription of TERT results in an increase in telomerase activity in the cancer cells,7 which ultimately enables them to avoid replicative senescence caused by telomere loss. BRAF p.V600E is a common variant occurring in several tumors, including melanoma, colorectal carcinoma, papillary thyroid carcinoma, hairy cell leukemia, Langerhans cell histiocytosis, and anaplastic thyroid carcinoma.8 This variant leads to the constitutive activation of the B-Raf kinase, which induces the aberrant activation of the MEK/ERK signaling pathway in cancer cells.9 BRAF and TERT promoter variants occur in about 20 percent and 40 percent of anaplastic thyroid carcinomas, respectively.10,11 Additionally, in papillary thyroid carcinomas the coexistence of BRAF and TERT promoter variants is associated with a significantly increased tumor recurrence rate and worse survival outcome.11
In summary, these two case studies demonstrate how NGS testing of tumors can help determine a definitive diagnosis when morphological and immunohistochemical findings alone are insufficient.
-
- Acosta AM, Adley BP. Predicting the behavior of perivascular epithelioid cell tumors of the uterine corpus. Arch Pathol Lab Med. 2017;141(3):463–469.
- Lae M, Gardrat S, Rondeau S, et al. MED12 mutations in breast phyllodes tumors: evidence of temporal tumoral heterogeneity and identification of associated critical signaling pathways. Oncotarget. 2016;7(51):84428–84438.
- Turunen M, Spaeth JM, Keskitalo S, et al. Uterine leiomyoma-linked MED12 mutations disrupt mediator-associated CDK activity. Cell Rep. 2014;7(3):654–660.
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16(3):155–166.
- Tsuyoshi H, Yoshida Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018;109(6):1743–1752.
- Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185.
- Borah S, Xi L, Zaug AJ, et al. Cancer. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347(6225):1006–1010.
- Loo E, Khalili P, Beuhler K, Siddiqi I, Vasef MA. BRAF V600E mutation across multiple tumor types: correlation between DNA-based sequencing and mutation-specific immunohistochemistry. Appl Immunohistochem Mol Morphol. 2018;26(10):709–713.
- Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther. 2011;10(3):385–394.
- Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368(7):684–685.
- Alzahrani AS, Alsaadi R, Murugan AK, Sadiq BB. TERT promoter mutations in thyroid cancer. Horm Cancer. 2016;7(3):165–177.
Kiavash Garakani is a graduate student in radiology and biomedical imaging, University of California, San Francisco. Dr. Devine is an assistant professor of pathology, and Dr. Ruiz-Cordero is assistant professor of pathology—both in the Department of Pathology, University of California, San Francisco.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
