CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Hackensack University Medical Center and Rutgers New Jersey Medical School. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Maciej Kabat, MD; Anja Jones, MD
Xiangyang Li, MS; Tatyana Feldman, MD
Xiao Yang, MD; Jennifer Zepf, DO
Kar Chow, MD; Pritish Bhattacharyya, MD
April 2024—Primary cutaneous marginal zone lymphoma (PCMZL) is a newly recognized, distinctive subtype of non-Hodgkin’s lymphoma. This low-grade lymphoma predominantly presents as papules or nodules within the skin of middle-aged adults. Formerly grouped under the extranodal marginal zone lymphoma (EMZL) category, the World Health Organization’s fifth edition classification of hematolymphoid tumors now recognizes PCMZL as a distinct entity.1
The pathogenesis of PCMZL has not been completely elucidated, but it is believed to be associated with chronic antigenic stimulation.2,3 PCMZL is generally classified into two subcategories: non-class switched and heavy-chain class-switched. The non-class switched subtype typically arises in the context of chronic Th1-type inflammation, often associated with Borrelia burgdorferi infection. In contrast, the heavy-chain class-switched subtype arises from chronic Th2-type inflammation.4,5 Associations between PCMZL and Borrelia burgdorferi have been observed, yet no definitive evidence connects PCMZL with viral etiologies such as Epstein-Barr virus, HIV, hepatitis virus, or autoimmune diseases. Patients typically present with papules, plaques, or nodules often localized on the trunk, extremities, or scalp.
Histologically, PCMZL is characterized by nodular to diffuse infiltrates of small lymphocytes, lymphoplasmacytoid, and plasma cells mixed with centroblasts and numerous reactive T lymphocytes.6 Immunohistochemical staining of biopsies is crucial for diagnosis, as flow cytometry can be inconclusive due to the challenge of isolating the malignant cell from the reactive process. These neoplastic cells typically express CD20, CD22, CD79a, and BCL2 and are negative for CD3, CD5, CD10, and BCL6. The reactive germinal centers express BCL6 but not BCL2. These cells frequently express polyreactive, somatically mutated immunoglobulin heavy chain genes.7 CD5 and cyclin D1 are useful in differentiating PCMZL (CD5– and cyclin D1–) from mantle cell lymphoma (CD5+ and cyclin D1+) and small lymphocytic leukemia (CD5+ and cyclin D1–).
This case report presents a challenging instance of PCMZL of the scalp, initially misdiagnosed as reactive lymphoid tissue. After the lesion recurred 18 months later, we used molecular markers, specifically immunoglobulin heavy chain variable, diversity, joining (IgH-VDJ) polymerase chain reaction, to detect identical clones in both samples. This finding led to a definitive diagnosis of PCMZL, highlighting the vital role of molecular technology in diagnosing malignant lymphoproliferative diseases, especially when characteristic morphologic and immunophenotypic changes are absent or inconclusive in flow cytometry and/or immunohistochemistry studies.
Case. The patient was a male in his early 70s who had a long-standing subcutaneous nodule in the occipital region of his scalp. The nodule, persisting for more than a decade, had remained stable but became increasingly bothersome. The patient reported no systemic symptoms, such as fever, chills, weight loss, or lymphadenopathy. Physical exam revealed a 2-cm movable, firm nodule in the left posterior occipital region. The skin covering the nodule was not erythematous. There was no cervical lymphadenopathy. Laboratory analyses were within normal limits: hemoglobin, 15.9 g/dL (ref. 13.0–17.0 g/dL); WBC, 8.2 × 103/μL (ref. 4.0–11.0 × 103/μL) with 16 percent lymphocytes (ref. 13–43 percent) and 73 percent neutrophils (ref. 40–75 percent); platelets, 160 × 103/μL (ref. 135–430 × 103/μL); creatinine, 0.6 mg/dL (ref. 0.3–1.3 mg/dL). Serological tests for various infections and autoimmune markers including HIV, hepatitis B and C, CMV, EBV, Lyme disease, chlamydia, ANA, and rheumatoid factor were negative or within normal ranges.
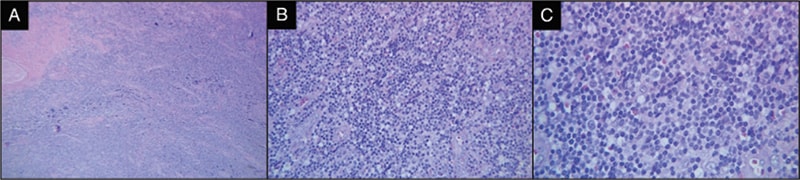
Fig. 1. A) Low-power H&E of the initial subcutaneous nodule on the occipital region of the scalp. B) H&E highlighting the distortion of nodal architecture by infiltration of small mature atypical lymphocytes. C) H&E highlighting small lymphocytes in a background of histiocytes.
The patient opted for surgical excision of the nodule. Initial histopathological analysis revealed dense fibrous tissue with a nodular infiltrate of atypical lymphoid cells, predominantly small, mature lymphocytes (Figs. 1 and 2). Reactive follicles were observed, and the infiltrate appeared to entrap nerves. Given the suspicion of an atypical B-cell proliferation, further consultation was sought from the National Cancer Institute. Immunohistochemical staining revealed a mixed population of B cells (CD20+, PAX-5+, BCL2+) and T cells (CD3+, CD5+, CD43+). There were vague nodules noted with BCL6+, CD20+, and OCT2+ cells, with Ki-67 activity less than five percent. Notably, the follicular dendritic meshwork was partially disrupted, as indicated by CD21 staining (Fig. 2). The plasma cells had no light chain restriction based on kappa/lambda immunohistochemistry. The tests for CD30, EBER-ISH, MUM1, CD10, IgD, CD5, and cyclin D1 were negative. Comprehensive lymphoma workup, including peripheral blood flow cytometry and serum protein studies (SPEP, SIFE, and β-2-microglobulin), did not indicate a lymphoproliferative disorder. Invivoscribe IgH-VDJ gene rearrangement kit by PCR (Invivoscribe, San Diego) was used on formalin-fixed, paraffin-embedded tissue, yielding small monoclonal spikes in the framework-2 and framework-3 regions, suggesting a potential oligoclonal or reactive process (Fig. 3).
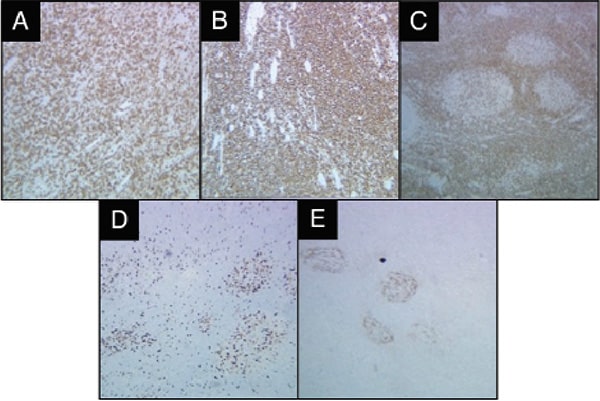
Fig. 2. Immunohistochemical staining of the original lesion. CD3 stain (A) highlights the background reactive T lymphocytes. The neoplastic cells are positive for B-cell markers CD20 (B) and BCL2 (C). The Ki-67 showed a low proliferation rate (D). The follicular dendritic meshwork is partially disrupted, highlighted by CD21 (E).
Given the nodule’s stability over a decade, absence of systemic disease, and atypical histological findings, the lymphocytic proliferation was deemed to be a reactive process. Moreover, the patient had undergone complete excision of the nodule, which was considered curative if the process was malignant.
About 18 months later, the patient returned with a recurrence of the nodule in the same region. A weeklong course of azithromycin led to mild subjective improvement. However, a PET scan revealed two adjacent fluorodeoxyglucose-avid (FDG-avid) cervical lymph nodes and increased avidity at the previous excision site. A core biopsy of the recurrent occipital nodule was obtained, but due to inadequate fixation, the specimen exhibited architectural distortion, numerous small lymphocytes with infiltration of the surrounding fibroadipose tissue, fibrosis, and crush artifact. Despite these challenges, the pathology report yielded results similar to the prior report (Fig. 4). The lymphoid cells were positive for CD20, CD79a, PAX-5, CD43, and BCL-2 but negative for CD3, CD5, CD68, CD163, and cyclin D1. The proliferation index as determined by Ki-67 was less than 10 percent. Flow cytometry did not reveal any evidence of a B-cell clonality. It showed that 44 percent of the cell population consisted of B cells with no distinct clonality, while 56 percent comprised reactive T cells with no loss of pan-T-cell markers.
Further analysis of IgH-VDJ rearrangement by PCR in the recurrent lesion revealed identical small monoclonal spikes at regions base pair 254 and 291 in framework region 2 and base pair 110 and 154 in framework region 3 (Fig. 3). These findings confirmed the persistence of the same monoclonal B cells, consistent with recurrence and progression of PCMZL. Following this diagnosis of relapsed PCMZL (cyclin D1–/CD5–), the patient began six weeks of rituximab therapy in addition to involved-site radiation therapy. Since the patient’s therapy, the nodule resolved and has not recurred.
Discussion. Diagnosing low-grade B-cell lymphoma can be a challenge for pathologists, particularly in cases where flow cytometric evaluation is unavailable or clonality cannot be detected due to background polyclonal B cells. In this case, the histological examination did not exhibit the typical characteristics of B-cell lymphoma, leading to a delayed diagnosis. Initially, this PCMZL was diagnosed as a reactive process.
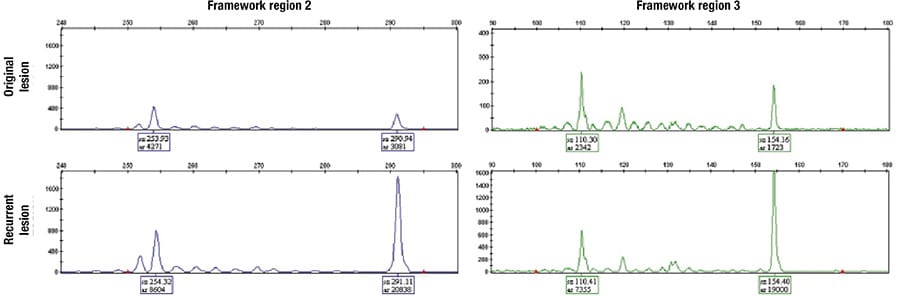
Fig. 3. IgH-VDJ rearrangement by PCR in the original and recurrent lesion revealed identical small monoclonal spikes at regions base pair 254 and 291 in framework region 2 and base pair 110 and 154 in framework region 3.
Upon recurrence, the core biopsy morphology from the recurrent lesion mirrored that of the initial specimen. Flow cytometry failed to demonstrate clear evidence of lymphoproliferative disorder. Crucially, molecular analysis, specifically the IgH-VDJ gene arrangement by PCR, revealed consistent monoclonal spikes in the FR2 and FR3 regions, identical to the previous lesion. This molecular consistency in combination with the PET scan findings was crucial in establishing the neoplastic nature of the lymphocyte population, thus confirming the neoplastic nature of the cell population in question and the diagnosis of PCMZL.

Fig. 4. A) Low-power H&E of the recurrent lesion demonstrates histology similar to the prior lesion, consisting of an infiltrate of small lymphocytes in a background of histiocytes. B) Immunohistochemical stain CD20 highlights the neoplastic B cells. C) The follicular dendritic meshwork is almost absent on CD21 immunohistochemical stain.
PCMZL generally has excellent five-year overall survival exceeding 95 percent. However, cutaneous relapses are common in patients with multifocal skin lesions and rarely may transform into an aggressive, blastic phenotype with dissemination beyond the skin.3,8–10 Treatment varies based on the lesion’s location and the disease stage. For isolated lesions, first-line management typically involves expectant watchful waiting and addressing the underlying inflammatory causes. However, in cases exhibiting high-risk features, more aggressive interventions such as surgery, radiation, chemotherapy, immunotherapy, or interferon therapy may be warranted.
This case highlights the importance of molecular techniques in establishing the diagnosis of a histologically challenging case of cutaneous PCMZL. The distinction between the reactive lymphoid process and neoplastic condition highlights the need for advanced molecular tools for accurate diagnosis.
- Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748.
- Rijlaarsdam U, Bakels V, van Oostveen JW, et al. Demonstration of clonal immunoglobulin gene rearrangements in cutaneous B-cell lymphomas and pseudo-B-cell lymphomas: differential diagnostic and pathogenetic aspects. J Invest Dermatol. 1992;99(6):749–754.
- Hoefnagel JJ, Vermeer MH, Jansen PM, et al.; Dutch Cutaneous Lymphoma Working Group. Primary cutaneous marginal zone B-cell lymphoma: clinical and therapeutic features in 50 cases. Arch Dermatol. 2005;141(9):1139–1145.
- van Maldegem F, van Dijk R, Wormhoudt TAM, et al. The majority of cutaneous marginal zone B-cell lymphomas expresses class-switched immunoglobulins and develops in a T-helper type 2 inflammatory environment. Blood. 2008;112(8):3355–3361.
- Edinger JT, Kant JA, Swerdlow SH. Cutaneous marginal zone lymphomas have distinctive features and include 2 subsets. Am J Surg Pathol. 2010;34(12):1830–1841.
- Willemze R, Kerl H, Sterry W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997;90(1):354–371.
- Craig VJ, Arnold I, Gerke C, et al. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood. 2010;115(3):581–591.
- Fink-Puches R, Zenahlik P, Bäck B, Smolle J, Kerl H, Cerroni L. Primary cutaneous lymphomas: applicability of current classification schemes (European Organization for Research and Treatment of Cancer, World Health Organization) based on clinicopathologic features observed in a large group of patients. Blood. 2002;99(3):800–805.
- Tsukamoto N, Kojima M, Uchiyama T, et al. Primary cutaneous CD5+ marginal zone B-cell lymphoma resembling the plasma cell variant of Castleman’s disease. Case Report. APMIS. 2007;115(12):1426–1431.
- Servitje O, Muniesa C, Benavente Y, et al. Primary cutaneous marginal zone B-cell lymphoma: response to treatment and disease-free survival in a series of 137 patients. J Am Acad Dermatol. 2013;69(3):357–365.
Dr. Kabat is in the Department of Internal Medicine; Xiangyang Li and Drs. Yang, Zepf, Chow, and Bhattacharyya are in the Department of Pathology; and Dr. Feldman is in the Division of Lymphoma, John Theurer Cancer Center—all at Hackensack University Medical Center, Hackensack, NJ. Dr. Jones is in the Department of Pathology and Laboratory Medicine, Rutgers New Jersey Medical School, Newark. Drs. Kabat and Jones contributed equally to this report.

 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
