CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from PacificDx clinical laboratory, Irvine, Calif. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Shelly Gunn, MD, PhD
Mathew W. Moore, PhD
Philip D. Cotter, PhD
August 2021—Dermatitis herpetiformis (DH) is an autoimmune skin disorder associated with celiac disease and characterized by pruritic, blistering lesions mainly on the elbows, knees, buttocks, lower back, and scalp. Immunopathogenesis is attributed to IgA TG3 antibody immune complexes in the papillary dermis originating from subclinical celiac disease in the gut.1 Onset occurs at any age, but DH is diagnosed most often in young adults and rarely in children. Individuals of northern European heritage represent the most frequently affected population for DH, with an estimated incidence reported by the National Organization for Rare Disorders of 75.3 per 100,000 people.2 First-degree family members of patients with DH are at increased risk for both DH and celiac disease.3
The cause of DH and celiac disease is multifactorial and includes a strong genetic component, specifically the presence of genetic risk haplotypes HLA-DQ2 and/or HLA-DQ8 encoded by variants of the HLA-DQA1 and HLA-DQB1 genes on chromosome 6.4 Diagnosis of DH can be made in patients with dermatologic and/or GI-related symptoms using perilesional skin biopsy for direct immunofluorescence microscopy to reveal pathognomonic IgA depositions in the papillary dermis.1 Diagnosis is supported by IgA anti-tissue transglutaminase (tTG) antibody serological testing and villous atrophy identified with endoscopic small bowel biopsy.5
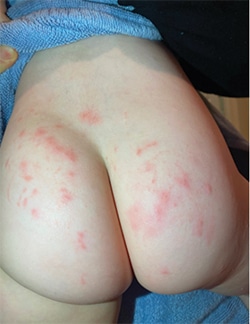
Fig. 1. Bilateral symmetrical polymorphic rash with excoriated blisters on both buttocks.
Tissue biopsy and tTG antibody testing for specific IgA class circulating antibodies are accurate for diagnosing DH and celiac disease when the patient is consuming dietary gluten to trigger active disease. Traditional confirmatory testing has a high false-negative rate in patients who have eliminated gluten from their diet resulting in quiescent disease. For confirmation of DH and celiac disease in “gluten free” patients, some physicians have used for diagnostic purposes a “gluten challenge,” in which the patient adds gluten back into the diet for four to eight weeks to reactivate disease in preparation for biopsy and antibody testing.6 This approach is disruptive for asymptomatic individuals, however, and needlessly invasive if they do not carry the DQ2 or DQ8 genes. In the case we report here, we discuss the use of next-generation sequencing as an accurate, one-time, noninvasive, celiac genetic risk assessment screening for the family of a child in remission from DH following elimination of gluten from his diet.
Case. A previously healthy 21-month-old child presented with a chronic symmetrical blistering pruritic rash covering both buttocks and mild GI symptoms that appeared shortly after introduction of solid foods including wheat products (Fig. 1). The rash persisted for several weeks and was evaluated via pediatric telemedicine because of the COVID-19 lockdown. The presumptive diagnosis was allergic reaction to a cat (even though the family did not own one), and topical steroids were initiated without improvement. His grandmother, an anatomic and clinical pathologist, recognized the characteristic DH lesions and suggested trial of a gluten-free diet. She had a high index of suspicion because of her son’s (the child’s father) history of chronic GI symptoms, which had never been diagnosed as celiac disease. Within a few weeks of eliminating gluten the child was completely asymptomatic. Serologic tTG and endomysial antibody testing performed after dietary gluten was eliminated was found to be negative for circulating IgA.
The family is of northern European origin and due to concern for the patient’s two asymptomatic siblings (ages four years and 10 months), family celiac genetic health risk studies were ordered.7 The testing was performed using PacificDx’s Gluten ID test, a next-generation sequencing assay designed to analyze variants associated with HLA-DQA1 and HLA-DQB1 genes for identification of haplotypes known to fall within a spectrum of risk for celiac disease. The test analyzes all possible celiac genetic risk haplotypes supported by level one (the highest) level of evidence. If none of the celiac risk-associated haplotypes are detected, the result has a negative predictive value for celiac disease of greater than 90 percent.
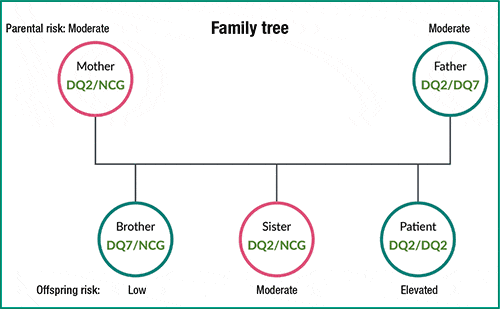
Fig. 2. Family tree showing inheritance of celiac genetic risk alleles.
Materials and methods. The laboratory extracted DNA from buccal cell swabs collected at home by all five family members and analyzed it using Illumina MiSeqDx technology. HLA-tagging celiac risk single nucleotide polymorphisms for DQ2.5, DQ8, DQ2.2, and DQ7 were amplified and sequenced at a minimum of 40× coverage. The test identified heterozygous and homozygous combinations of the celiac risk alleles for each family member, and these were reported within a spectrum of risk from low to elevated. When none of the celiac risk alleles were identified, the results were reported as nonceliac genetics (NCG).
Results. The homozygous DQ2/DQ2 Gluten ID associated with elevated celiac disease risk was identified in the patient. Each of his parents was found to carry one DQ2 and his father also had a DQ7 allele, placing both parents at moderate risk. His four-year-old sister inherited the paternal DQ2 and maternal NCG, placing her at moderate risk also. His younger brother inherited DQ7 from the paternal chromosome and a maternal NCG, placing him on the lowest end of the risk spectrum7 (Figs. 2 and 3).
Discussion. Approximately 30 to 40 percent of the world population carries specific allelic combinations of DQ2/DQ8, placing them on a spectrum of risk for celiac disease. It is well supported in the medical literature that the presence of DQ2/DQ8 genes is necessary but not sufficient for development of celiac disease, and the negative predictive value of negative DQ2/DQ8 test results is greater than 90 percent. Thus, an individual who tests negative for celiac risk alleles is highly unlikely to have celiac disease or benefit from invasive diagnostic procedures, except in the rare scenario of high clinical suspicion of celiac disease in the presence of negative genetics.
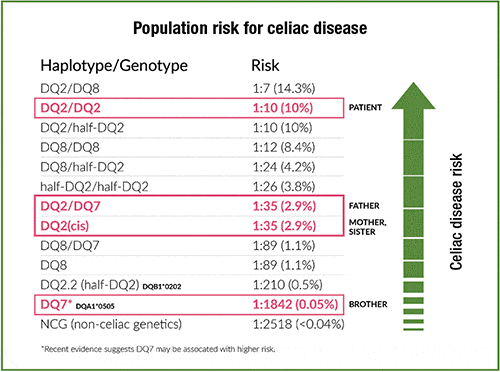
Fig. 3. The family’s genetic risk shown within the population spectrum of risk for celiac disease. The figure is based on genetic risk gradient data from Pietzak, et al., and Megiorni, et al.8,9 The half-DQ2 (DQB1*0202) and DQ7 (DQA1*0505) haplotype/genotypes encode DQ2 in the trans configuration if inherited together from maternal and paternal chromosomes. The celiac genetic risk for DQ2 trans is similar to DQ2 in the cis configuration where DQA1*0501 and DQB1*0201 genes are inherited as the DQ2.5 haplotype on the same chromosome 6 from a single parent.10
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
