No definite invasion should be identified in a diagnosis of papillary carcinoma, Dr. Li said. “If you can see invasive carcinoma, call it invasive carcinoma.” More accurate classification of papillary carcinoma without invasion can be deferred to excision, he said, “because treatment certainly will be the same.”
Sixty to 75 percent of phyllodes tumors are benign, with a 10 to 17 percent recurrence rate and no metastatic potential. Fifteen to 26 percent are borderline; they have a 14 to 25 percent recurrence rate and virtually no metastatic potential. And eight to 20 percent of phyllodes tumors are malignant, with a 23 to 30 percent recurrence rate and low metastatic potential.
Classic-type fibroadenoma morphology is straightforward, “with a stromal cellularity similar to the perilobular stroma in the surrounding benign breast tissue,” he said. But some may show unusual features. They may be involved by atypical epithelial proliferation, carcinoma in situ, or invasive carcinoma. And some have myxoid changes in the stromal component, he said, noting myxoid fibroadenomas are sometimes associated with Carney complex, so for this he recommends clinical correlation for Carney complex.
Rarely, fibroadenomas may have a focal and permeable border, Dr. Li said. They also may be involved by pseudoangiomatous stromal hyperplasia or smooth muscle differentiation. Typically, they do not contain adipose tissue, but infrequently they can have lipomatous metaplasia. Relatively large fibroadenomas, in particular, he noted, can have focal infarction. Some fibroadenomas have more stromal proliferation; at the other end of the spectrum there is more tubular proliferation.
“The problem we often face is how to differentiate cellular fibroadenomas from phyllodes tumors in core biopsies,” he said. Both have well-demarcated borders. Cellular fibroadenomas often have increased stromal cellularity; benign and borderline phyllodes tumors can have mild to moderately increased stroma. Neither fibroadenomas nor benign phyllodes tumors have severe stromal atypia, and both have low mitotic count (borderline and malignant phyllodes have a higher mitotic count; 5–9/10 HPF and ≥ 10/10 HPF, respectively). And neither fibroadenomas nor benign phyllodes tumors have stromal overgrowth or malignant heterologous components (malignant phyllodes tumors have stromal overgrowth and may have malignant heterologous components).
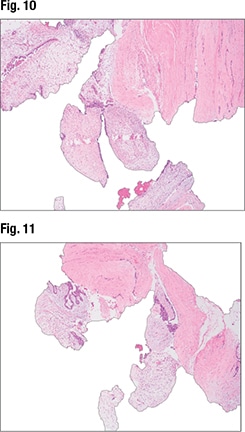
The third case Dr. Li presented is that of a 50-year-old female who presented with a 12-cm well-circumscribed breast mass. In the core biopsy, “we can see biphasic proliferation with epithelial proliferations and stromal proliferation. The biopsy is kind of fragmented,” and there’s some stromal heterogeneity in terms of the cellularity, with some areas showing more stromal cellularity and others less (Fig. 9). At higher power (Fig. 10), intratumoral heterogeneity can be seen. In Fig. 11, tissue fragmentation can be seen. Under higher magnification (Fig. 12), “there’s no severe cytologic atypia and virtually no mitosis in the stromal components.”

The lesion was initially diagnosed as a fibroadenoma with myxoid changes. On excision it looked like what was seen in the core biopsies: biphasic proliferation with stromal and epithelial components, some intratumoral heterogeneity, and some areas with more stromal cellularity and others with less. Under high magnification the background stroma are seen to have focal myxoid changes, but there’s no severe cytologic atypia. In other areas of the tumor, however, “we see stromal components without epithelial components. So this is stromal overgrowth. And some areas show increased mitosis.”
Six months later, “this patient had metastasis to the lung,” he said. The true diagnosis was malignant phyllodes tumor.
“So what features in a core biopsy are not typical of a fibroadenoma?” Large tumor size is one. Intratumoral stromal heterogeneity is another, as is tissue fragmentation, which is sometimes associated with phyllodes tumor. Several studies have noted the morphological features in core biopsy that are associated with phyllodes tumor diagnosis in excision (in addition to fragmentation), he said: stromal overgrowth, ≥ 2 mitoses/10 HPF, marked stromal hypercellularity, stromal pleomorphism, heterogeneity, infiltrating border, adipose tissue with stroma, large tumor size, and age ≥ 50 years.
“Of all these features, be careful of stromal pleomorphism,” he said. Stromal giant cells are benign reactive stromal cells that can be seen in fibroepithelial and other breast lesions (and lesions from other organs). Studies have found they do not increase the risk of recurrence of fibroepithelial lesions. They are generally negative for cytokeratin staining and mitotically inactive. “However, you can see occasional mitosis of these stromal giant cells. So be careful with the stromal giant cells,” Dr. Li cautioned. “Do not overcall these lesions.”
“It’s okay to call fibroepithelial lesion and recommend excision if you’re not confident to differentiate fibroadenoma from phyllodes tumor. But if the lesion shows all the classic features of a fibroadenoma, call fibroadenoma. Because if you call every lesion a fibroepithelial lesion, that may result in unnecessary surgeries.” And differentiating cellular fibroadenoma from phyllodes tumor can be difficult even in surgical specimens, he noted.

Lawton TJ, et al., reported significant interobserver variability even among pathologists who specialize in breast pathology in distinguishing between cellular fibroadenomas and phyllodes tumors. In only two of 21 selected cases of fibroepithelial lesions sent in consultation that were challenging to classify as fibroadenoma or phyllodes tumor was there uniform agreement (Lawton TJ, et al. Int J Surg Pathol. 2014;22[8]:695–698).
Two key features differentiate cellular fibroadenoma from benign phyllodes tumor, according to consensus opinion: a well-developed leaf-like structure (Fig. 13) and stromal cellularity. The key feature is the former because there can be overlap with the latter.
Cellular fibroadenomas, classic-type fibroadenomas, and benign phyllodes tumors harbor MED12 and RARA mutations, Dr. Li said. TERT promoter mutations occur more frequently in benign phyllodes tumor than in cellular and classic-type fibroadenoma. “So there could be progression from fibroadenoma to phyllodes tumor,” Dr. Li said, when a fibroadenoma is hit by a TERT promoter mutation.

The recurrence rate of benign phyllodes tumors varies from study to study, Dr. Li noted. “The question we usually face in practice is, ‘Do these patients need wide margins?’” In one multicenter study that evaluated the margin status to recurrence rate in 550 phyllodes tumors, 1.7 percent of benign phyllodes tumors with positive margins recurred. When there was a negative but narrow margin (< 2 mm), 2.7 percent recurred. And in the tumors with relatively wide negative margins (≥ 2 mm), 1.1 percent recurred (Rosenberger LH, et al. J Clin Oncol. 2020;39[3]:178–189). The study’s authors concluded that recurrence is not associated with wider negative margins, final margin status (positive versus negative), surgery type, mitosis, tumor border, or age. “So it seems from the current studies that wide margins are not needed for benign phyllodes tumors,” Dr. Li said, “and treatment should be personalized.” (The study also reports the local recurrence rates of borderline and malignant phyllodes tumor.)
In practice, Dr. Li said, “we often face the situation of differentiating metaplastic carcinomas from malignant phyllodes tumors.” A DCIS or malignant carcinoma adjacent to a spindle cell lesion favors a metaplastic carcinoma. Cytokeratin, p63, and CD34 staining are helpful in differentiating the two. Metaplastic carcinomas typically are positive or focally positive for cytokeratin and p63 and negative for CD34. But some malignant phyllodes tumors can be focally positive for cytokeratin staining and p63, and some can be negative for CD34, he said. “So if CD34 is negative, it’s not that helpful. But if CD34 is positive, you can virtually rule out metaplastic carcinoma.”
Charna Albert is CAP TODAY associate contributing editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
