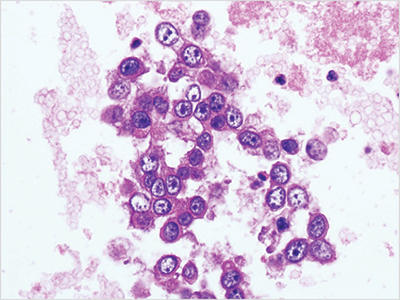
Fig. 4. Cellient automated cell block system preparation (ascitic fluid preparation, adenocarcinoma, hematoxylin and eosin stain, 40×).
The specimen is centrifuged at 3,000 rpm for five minutes. The supernatant is poured off and the sediment remains in the tube. HistoGel (HG) is liquefied by melting it on medium power in the microwave for five to 15 seconds. Enough HG to cover the sediment (about 0.5 mL) is added to the tube and mixed with the sediment. Allow HG to solidify (two to three minutes at room temperature or quicker if using a cooling block). The solidified HG sediment is dislodged from the bottom of the tube by squirting 10 percent neutral buffered formalin into the side of the tube. Remove cell block sediment and place in filter paper in a labeled cassette in formalin. The specimen is then processed routinely in the histopathology laboratory.
Care should be taken when facing and cutting the paraffin block because the tissue fragments may be right at the surface of the block.
Tissue coagulum clot method. This method allows a clot to form in the lumen of the fine needle aspiration tip. The clot is then transferred directly to formalin for fixation. The technique prevents the loss of diagnostic material.
To prepare cell blocks using the tissue coagulum clot method, the material within the 21-gauge/22-gauge cytology needle is gently pushed out using the wire stylet found with the needle kit, instead of using a syringe to expel the material into saline for cell block preparation. As the material exits the needle tip, it is collected onto a precut piece of filter paper with the needle tip directed in a tight circular motion to build up a cone-shaped coagulum of tissue and blood mixture. The clot is slightly air-dried on the filter paper, mostly to ensure the coagulum is solid and the cellular elements are not dispersed in a liquid medium. The specimen is wrapped in tissue paper and placed in a cassette and container of formalin. The specimen is then processed routinely in the histopathology laboratory.
Formalin or alcohol vapor method. A specimen is fixed using formalin or alcohol fumes. This method requires no additional equipment and minimal reagents. The material is expelled from a fine needle aspiration to form a small clot on the inside of a lid of a universal specimen container. The lid is inverted and a ball of tissue paper is pushed into the bottom of the container. About 2 mL of formalin is added to the container and soaks into the tissue paper.
If alcohol is used, two isopropyl alcohol phlebotomy swabs are placed in the bottom of the container.
The container is left in the inverted position for at least six hours at room temperature. By this time the specimen has been fixed by formalin or alcohol vapor and has become solid. It is taken off the lid by adding formalin to break the suction between specimen and lid. The specimen should be placed in a cassette and formalin added as soon as it is removed from the lid. The specimen is then processed routinely in the histopathology laboratory.
Cell block technique for cell pellet or tissue fragments—method No. 1 (using 95 percent alcohol). This commonly used method uses alcohol for centrifuging the specimen that is submitted in a preservative alcohol-based medium.
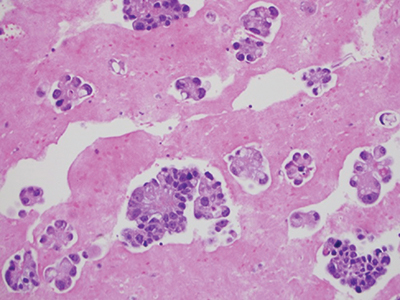
Fig. 5. Cell block technique for cell pellet or tissue fragments—method No. 2 using saline, RPMI, or formalin for collection, hematoxylin and eosin stain, 20×.
Label a 50-mL conical centrifuge tube with multiple patient identifiers. Decant up to 50 mL of specimen into the labeled tube. Store an aliquot (if necessary) for ancillary testing. Centrifuge specimen with fixative (95 percent alcohol) or preservative medium (e.g. CytoLyt) for 10 minutes (2,500 rpm). Decant the supernatant and thoroughly vortex the specimen (determine if you need a second centrifugation step to lyse grossly bloody specimens or hardened/compact cell pellet). Add aliquot of 95 percent alcohol to specimen, mix thoroughly, centrifuge again. Decant alcohol completely.
At this point, based on the size of the cell pellet, you can choose the most appropriate method. If the cell block pellet is very small and/or sediment is scant or does not pack well, use an alternative technique such as HistoGel or agar.
Remove cell pellet from the conical tube with a spatula; section as necessary to match the width of a nickel. Place in a tissue cassette. Place a sponge with a hole cut out of the center in the bottom of the labeled tissue cassette. Sponges may not be necessary with a large, well-solidified cell pellet.
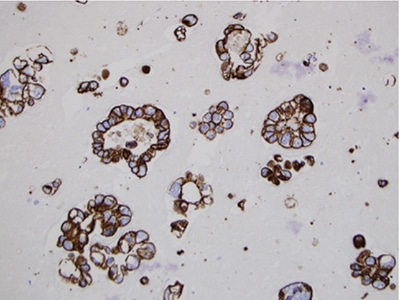
Fig. 6. Cell block technique for cell pellet or tissue fragments—method No. 2, MOC 31 immunocytochemical stain, 20×.
Place an appropriate size piece of lens paper on top of the blue sponge. Place the cell block section on top of the lens paper, centered in cassette. Place a second piece of lens paper, at a 45-degree angle, on top of the cell block section. Place a second sponge with a hole cut out of the center on top of the second piece of lens paper. Securely snap the cassette cover in place and place the cassette in formalin. The specimen is then processed routinely in the histopathology laboratory.
Cell block technique for cell pellet or tissue fragments—method No. 2 (using saline, RPMI, or formalin for collection). This method is commonly used for cell block preparation (Figs. 5 and 6).
Transfer material to a 50-mL conical centrifuge tube and label with multiple patient identifiers. Set the centrifuge to run at 2,400 rpm for five minutes to achieve a concentrated cell button. Pour off supernatant. The button may not be cohesive and it may start to fall apart as the supernatant is being removed. Should this happen, equal parts neutral buffered formalin can be added to help fix the specimen. This mixture should sit for 30–60 minutes to allow material to set. The hardened cell button is then removed with a metal spatula by loosening the edges until it breaks free of tube. The button is then placed on histology tissue paper that has been moistened with neutral buffered formalin. Tissue paper is folded up around the button and placed in a histology cassette.
 Once all steps are complete, the cassette and its contents are put in a specimen cup filled with buffered formalin, which should cover the cassette entirely. Allow the specimen to fix for at least 30 minutes or longer. The specimen is then processed routinely in the histopathology laboratory.
Once all steps are complete, the cassette and its contents are put in a specimen cup filled with buffered formalin, which should cover the cassette entirely. Allow the specimen to fix for at least 30 minutes or longer. The specimen is then processed routinely in the histopathology laboratory.
If the cell block pellet is very small and/or sediment is scant or does not pack well, use an alternative technique such as HistoGel or agar. Sponges may not be necessary with a large, well-solidified cell pellet.
- Castro-Villabon D, Avello Y, Ruiz N, Rodriguez-Urrego PA. Implementation of routine thromboplastin-plasma cell block technique in the evaluation of non-gynecologic specimens. J Microscopy Ultrastructure. 2014;2:177–181.
- Gill GW. Cell block preparation. In: Cytopreparation: Principles and Practice, Essentials in Cytopathology. New York, NY: Springer-Verlag; 2013.
- Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25(6):356–371.
- Khan S, Omar T, Michelow P. Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol. 2012;
29(3):177–182. - Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37(2):86–90.
- Mayall F, Chang B, Darlington A. A review of 50 consecutive cytology cell block preparations in a large general hospital. J Clin Pathol. 1997;50(12):985–990.
- Mayall F, Darlington A. The poor man’s cell block. J Clin Pathol. 2010;63(9):837–838.
- Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114(4):599–606.
- Varsegi GM, Shidham V. Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009;29:e1316.
- Varghese S, Martin A, Russell D, McMahon L, Fiscella J. Comparison of immunohistochemistry staining performed on cell blocks versus surgical blocks from the same tumor. Mod Pathol. 2017;30(S2):520A–521A.
- Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, Bonfiglio TA. Cellient automated cell block versus traditional cell block preparation: a comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol. 2011;39(10):730–736.
- Yung RC, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120(3):185–195.
- Zanchelli-Astran G, Wood M, Bibbo M. Making the diagnosis with only two levels of nongynecologic cell blocks as opposed to three is more cost effective. Diagn Cytopathol. 2010;38(5):374–376.
Dr. Rollins is director of the Outpatient Cytopathology Center, Johnson City, Tenn. Donna Russell is in the Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, NY. Both are members of the CAP Cytopathology Committee.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
