Donna K. Russell, MEd, CT(ASCP)HT, CFIAC
May 2022—The number of minimally invasive fine-needle aspirations requiring rapid on-site evaluation (ROSE) in the cytopathology laboratory has increased over the past decade. Laboratories have seen lower gynecologic volumes and an increase in both nongynecologic fine-needle aspiration biopsy and touch imprint samples. ROSE for patient care has proven value. Sample adequacy allows for a single visit and avoids having to make multiple attempts to provide material sufficient for all required testing, including flow cytometry, microbiology, cell block preparation for immunohistochemical and histochemical staining, and molecular testing.
The cytology laboratory provides ROSE for a variety of fine-needle aspiration samples in multiple locations within institutions and often at outreach clinics. Endoscopic ultrasound-guided FNAs are performed in the GI suite; endobronchial ultrasound-guided FNA and electromagnetic navigation bronchoscopy can be performed in the pulmonary suite or the operating room. Radiologist-performed FNAs can be done in multiple rooms depending on the imaging technique. Cancer centers and endocrinology suites provide ROSE for patient care, and this service can be provided at an array of locations, which has prompted the move from traditional on-site assessment to telecytology platforms. Users of telecytology have shown satisfactory competence as defined by concordance in various studies.1
Telecytology allows for adequate reimbursement for each episode of adequacy during ROSE. High-resolution digital imaging technologies have made telecytology for ROSE a reality and make it possible for the pathologist to stay in the office and perform other tasks during ROSE downtime. Consensus on difficult cases can be obtained using telecytology.2-5 ROSE of minimally invasive cytology helps to avoid repeat procedures, reduces potential complications, and decreases health care costs.6
Telecytology can be sent via live image streaming, robotic live image streaming, or through static image transmission. Most practices with high volumes of ROSE use live image streaming. It makes it possible for the pathologist to review the entire slide and is more efficient than on-site evaluation.6 Several systems are on the market and additional videoconferencing systems are available. Telecytology robotic microscopes are suitable for ROSE and give the pathologist full control of the slide with magnification and focusing. Sirintrapun, et al., have reported the use of robotic microscopy for ROSE and found concordance between the preliminary adequacy assessment and the final assessment at 92.7 percent.7 This method can be useful in low-volume practices or where there is no on-site cytology expertise to demonstrate fields of interest for the pathologist performing the evaluation. Static image transmission is similar and used in low-volume settings. The images taken for assessment need to be accurate; thus a high level of expertise is required to select appropriate images for transmission.8 All three modes of transmission have been shown to be accurate with the use of telecytology.7,9-16
Technological advances, as well as laboratory staffing shortages and the pandemic, have all contributed to a shift from on-site evaluation to coverage by telecytology, and as cytopathology practices implement telecytology, it must be monitored for quality. The CAP has accreditation program checklist requirements for telepathology. Requirement GEN.52860 says “Telepathology services are included in the laboratory’s quality management system,” and it suggests “comparison to on-site evaluation” as an example of a metric relevant to telecytology.17 This section of the checklist is applicable to, but not limited to, anatomic pathology and cytopathology, hematopathology, cytogenetics, and other disciplines. It applies to all diagnostic applications—primary diagnosis, second opinion, remote FNA assessment, and frozen section interpretation.The laboratory’s telepathology system for any diagnostic purpose must be validated before it is implemented. GEN.50630 Telepathology System Validation (phase one) is consistent with implementation of new systems throughout the laboratory and with GEN.52920 Whole Slide Imaging System Validation/Verification.17 It reads as follows: “The laboratory validates telepathology systems used for clinical diagnostic purposes by performing its own validation studies, including approval for use by the laboratory director (or designee who meets CAP director qualifications) before the technology is used for the intended diagnostic purpose(s).” It says the validation process must emulate the “real-world clinical environment” and records of the validation must be retained. Other requirements involving patient and specimen identification call for a method to ensure correct patient identification of data files submitted for review.
Security of data transmission and patient confidentiality are critical. GEN.52842 Patient Confidentiality—Telepathology and Remote Data Assessment says security procedures must be in place and followed in particular when portable mobile devices are used in public places.17
Digital images used for diagnosis must be retained for 10 years if the original glass slides are not available (see ANP.12500).18 There is no retention requirement if original slides are available and readable for the 10-year required retention period.
New requirements will be added and revisions made to existing requirements as digital pathology, telepathology, and whole slide imaging advance.
Laboratories that implement telepathology for clinical use need to perform validation studies, and they should incorporate the intended clinical use of the system to be deployed,19 including telecytology for ROSE. McCarthy, et al., demonstrate validation of a telecytology system for on-site evaluation of FNA biopsies.20 The authors address the evaluation of the competency of users by looking at both intra- and interpathologist variability. All participating pathologists were tested with 10 random FNA cases, and each participant needed a passing rate of at least 90 percent.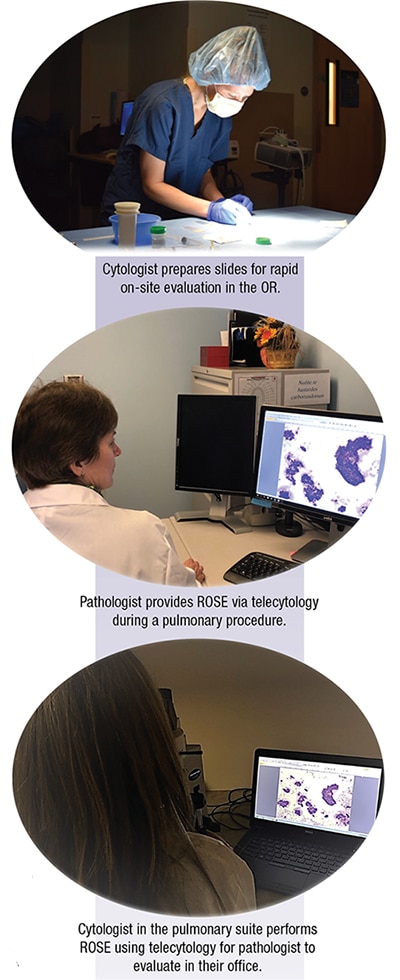 Monaco and Pantanowitz, in an editorial,1 commended authors for emulating a “real world” environment during their validation study. Cytotechnologists prepared slides and operated the microscope for telecytology, with pathologists at a remote location providing interpretation. They avoided bias in the study by using an acceptable six-week period for individual interpreters when assessing intraobserver variability. The number of cases evaluated was considered low; the recommended number of cases is at least 60 for validating whole slide images for primary diagnosis.19 The larger number of cases makes it possible for interpreters to overcome the learning curve.
Monaco and Pantanowitz, in an editorial,1 commended authors for emulating a “real world” environment during their validation study. Cytotechnologists prepared slides and operated the microscope for telecytology, with pathologists at a remote location providing interpretation. They avoided bias in the study by using an acceptable six-week period for individual interpreters when assessing intraobserver variability. The number of cases evaluated was considered low; the recommended number of cases is at least 60 for validating whole slide images for primary diagnosis.19 The larger number of cases makes it possible for interpreters to overcome the learning curve.
In a study by Trabzonlu, et al., on the differences in real-time images seen by different observers, sessions were arranged in which all observers evaluated cases at the same time.21 On-site operators were also involved in the validation because they play a crucial role in telecytology workflow. As a result, the study showed a significant difference in the adequacy concordance rate between the test and real case sets, which proves that observers and on-site operators benefit from the training portion and perform better in the validation phase.
Multiple studies of telecytology have shown diagnostic accuracy when compared with traditional ROSE. Some institutions have also included training, competency assessment, and internal validation in their studies. In a study by Green, et al., a telecytology QA process was developed to satisfy CAP requirements, monitor the diagnostic accuracy of telecytology, and identify problematic trends over time.22 Through a retrospective review, their program met CAP requirements and confirmed diagnostic accuracy over time.
Validation of a telepathology system for assessment should include a validation summary statement to include the equipment involved in telecytology, the start and completion date of the validation, as well as the location of the performed validation, and that the system is operated properly in accordance with requirements of the manufacturer and the cytopathology laboratory. All installation, operation, and performance requirements have to be met without deviation. System training with all staff cytotechnologists, fellows, and cytopathologists must be completed successfully. There should be blind parallel studies with the cytopathologists with at least 90 percent concordance for adequacy. The validation for telepathology needs to be concluded successfully; then the system is ready for FNA adequacy assessment. Deviations or errors with connectivity and the percent of concordance should be reported in the summary.
1. Monaco SE, Pantanowitz L. Telecytology value and validation: developing a validation and competency tool for telecytology. Diagn Cytopathol. 2015;43(1):1–2.
2. Pantanowitz L, Hornish M, Goulart RA. The impact of digital imaging in the field of cytopathology. Cytojournal. 2009;6:6.
3. Weinstein RS, Graham AR, Richter LC, et al. Overview of telepathology, virtual microscopy, and whole slide imaging: prospects for the future. Hum Pathol. 2009;40(8):1057–1069.
4. Williams S, Henricks WH, Becich MJ, Toscano M, Carter AB. Telepathology for patient care: what am I getting myself into? Adv Anat Pathol. 2010;17(2):130–149.
5. Marotti JD, Johncox V, Ng D, Gonzalez JL, Padmanabhan V. Implementation of telecytology for immediate assessment of endoscopic ultrasound-guided fine-needle aspiration compared to conventional on-site evaluation: analysis of 240 consecutive cases. Acta Cytol. 2012;56(5):548–553.
6. Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7(1):1–6.
7. Sirintrapun SJ, Rudomina D, Mazzella A, et al. Robotic telecytology for remote cytologic evaluation without an on-site cytotechnologist or cytopathologist: a tale of implementation and review of constraints. J Pathol Inform. 2017;8:32.
8. Thrall M, Pantanowitz L, Khalbuss W. Telecytology: clinical applications, current challenges, and future benefits. J Pathol Inform. 2011;2:51.
9. Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45.
10. Singh N, Akbar N, Sowter C, Lea KG, Wells CA. Telepathology in a routine clinical environment: implementation and accuracy of diagnosis by robotic microscopy in a one-stop breast clinic. J Pathol. 2002;196(3):351–355.
11. Ayatollahi H, Khoei A, Mohammadian N, et al. Telemedicine in diagnostic pleural cytology: a feasibility study between universities in Iran and USA. J Telemed Telecare. 2007;13(7):363–368.
12. Jialdasani R, Desai S, Gupta M, et al. An analysis of 46 static telecytology cases over a period of two years. J Telemed Telecare. 2006;12(6):311–314.
13. Sirintrapun SJ, Rudomina D, Mazzella A, Feratovic R, Lin O. Successful secure high-definition streaming telecytology for remote cytologic evaluation. J Pathol Inform. 2017;8:33.
14. Cai G, Teot LA, Khalbuss WE, et al. Cytologic evaluation of image-guided fine needle aspiration biopsies via robotic microscopy: a validation study. J Pathol Inform. 2010;1:4.
15. Heimann A, Maini G, Hwang S, Shroyer KR, Singh M. Use of telecytology for the immediate assessment of CT guided and endoscopic FNA cytology: diagnostic accuracy, advantages, and pitfalls. Diagn Cytopathol. 2012;40(7):575–581.
16. Khurana KK, Rong R, Wang D, Roy A. Dynamic telecytopathology for on-site preliminary diagnosis of endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. J Telemed Telecare. 2012;18(5):253–259.
17. College of American Pathologists. Laboratory general checklist. Sept. 22, 2021.
18. College of American Pathologists. Anatomic pathology checklist. Sept. 22, 2021.
19. Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722.
20. McCarthy EE, McMahon RQ, Das K, Stewart J III. Internal validation testing for new technologies: bringing telecytopathology into the mainstream. Diagn Cytopathol. 2015;43(1):3–7.
21. Trabzonlu L, Chatt G, McIntire PJ, et al. Telecytology validation: is there a recipe for everybody? Published online March 10, 2022. J Am Soc Cytopathol. doi:10.1016/j.jasc.2022.03.001
22. Green DM, Boivin ME, Everts RM, et al. Implementation and assessment of a telecytology quality assurance program. J Am Soc Cytopathol. 2021;10(2):239–245.
Donna K. Russell is supervisor, cytopathology residency, University of Rochester Medical Center in New York, and a CAP Cytopathology Committee member.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
