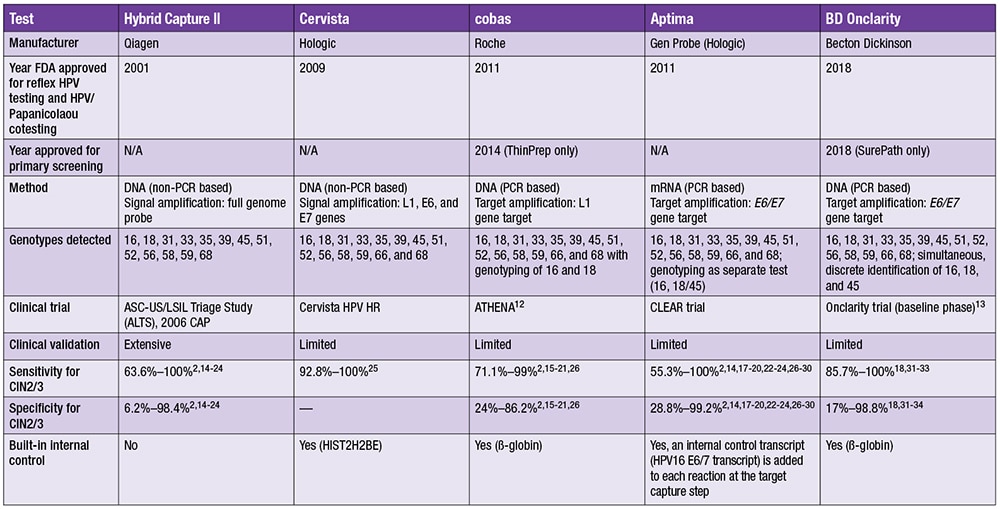
Table 1. Comparison of the 5 FDA-approved testing platforms (Abbreviations: N/A, not applicable; PCR, polymerase chain reaction. Reprinted from Journal of the American Society of Cytopathology, vol 8, Katrina L. Salazar, Daniel J. Duhon, Randall Olsen, Michael Thrall. A review of the FDA-approved molecular testing platforms for human papillomavirus, page 286, Copyright (2019), with permission from Elsevier.)
Pages: 1 2
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
