Shahla Masood, MD; Anwer Siddiqi, MD, MS
May 2022—Substantial progress has been made during the past several years in diagnosing and treating various illnesses. Advances in genetic and genomic science; imaging and localization devices; the use of minimally invasive diagnostic sampling procedures; diagnostic, prognostic, and predictive testing; and personalized therapeutic options—all have changed the pattern of the practice of medicine and how patient care is provided.
Pathologists and cytopathologists have played a central role in the realization of this progress and have been involved in developing, validating, and implementing new diagnostic and predictive testing modalities and ensuring their appropriate use. In addition, pathologists have been engaged not only in interpreting but also in performing minimally invasive procedures. New technologies have been optimized for the small sample sizes obtained from these procedures.
Minimally invasive diagnostic sampling procedures have reduced the numbers of open surgical biopsies and proved to be time-tested, convenient, cost-effective, and rapid. They’re designed to lessen the anxiety of patients with benign diseases. Rapid on-site evaluation (ROSE) has accelerated the diagnosis of malignancy and the plans for optimal patient therapy. This approach is an effective display of coordinated care among multiple disciplines involved in imaging, cell/tissue acquisition, and biopsy result interpretation.
Access to rapid interpretation of test results during minimally invasive diagnostic sampling procedures is critical to building trust between health care providers and patients. ROSE is similar to point-of-care testing, which provides a reliable means of characterizing the nature of a disease process and a sense of direction on what the next best step is for real-time personalized care—and a better patient experience.
At the beginning. Since the introduction of imprint cytology by Dudgeon and Patrick1 in 1927, its value during surgeries in evaluating specimens from a variety of organs has been described in the literature.2,3 It was initially designed for use during intraoperative consultations where it enhanced frozen section diagnostic accuracy up to 99.2 percent. In countries with limited resources (no availability of frozen section), imprint cytology is most helpful in the follow-up management planning of patient care.4,5 Later, imprint cytology was used in assessing the presence or absence of metastasis in sentinel lymph node biopsies and for evaluating lumpectomy surgical margins in breast cancer patients.6,7
We have also used imprint cytology and other cytologic preparations to assess hormone receptor status in breast cancer patients and in other samples.8,9 This approach is most suitable in cases of limited biopsy specimens and in effusions due to cancer metastasis. The requirement to provide information about biomarkers in tumors suitable for presurgical chemotherapy makes the use of cytologic preparation for these assessments appropriate and necessary.
Practice today. Aside from fine-needle aspiration biopsy and core needle biopsy, other sampling platforms such as endobronchial ultrasound-guided and endoscopic ultrasound biopsy have provided more opportunities to use ROSE. There is now sufficient evidence in the literature supporting the use of ROSE as an effective diagnostic tool in assessing various lesions at different body sites. Aside from adequacy assessment, it is now generally agreed that ROSE may allow appropriate triage of biopsy specimens for early planning of ancillary studies with rapid turnaround time in difficult-to-diagnose cases. In uncomplicated cases, ROSE provides an accurate diagnosis equivalent to that of a surgical biopsy and reduces the need for unnecessary passes. This practice, however, requires access to an on-site cytopathology service.10-19
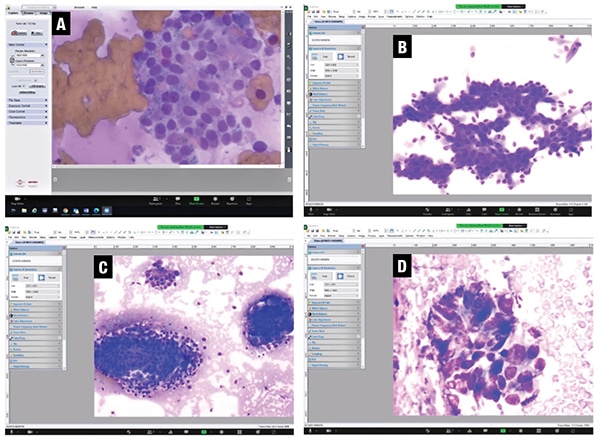
Fig. 1. Screen capture of the real-time video images viewed at the main hospital. A. Ultrasound-guided FNA biopsy smear of a thyroid lesion with benign Hurthle cells (DiffQuik stain, 20×). B. Ultrasound-guided FNA biopsy smear of a pancreatic head lesion with benign ductal cells (DiffQuik stain, 10×). C. CT-guided core imprint of a lung lesion with benign bronchial cells on left and a cluster of malignant cells on right (DiffQuik stain, 10×). D. Ultrasound-guided FNA biopsy smear of a pancreatic head lesion with adenocarcinoma (DiffQuik stain, 20×).
Minimally invasive biopsy procedures are often performed at a hospital at multiple locations inside and outside the institution such as radiology suites or in specialty dedicated clinics. Since these locations are not typically adjacent to the cytopathology laboratory, ROSE for these procedures and subsequently conveying specimen adequacy and preliminary diagnosis for the same may require a higher level of specialty service from cytopathology staff. Ultimately, telecytopathology has been found to be a reasonable solution in offering ROSE, for which demand is increasing at medical centers and in clinical practices.
A cytopathologist is critical to the effectiveness of the ROSE procedure, but the need for a cytopathologist to be present at multiple sites, and the unexpected delays and waits, can be a burden. Assessing adequacy, waiting for multiple passes, and providing a preliminary diagnosis on cytologic preparations are time-consuming. Therefore, with the current level of reimbursement, it is difficult for a cytopathologist to participate in the on-site evaluation process.20 As a result, cytotechnologists are now involved in the majority of ROSE procedures. Some providers such as interventional pulmonologists have reportedly learned enough cytology to be able to assess the adequacy of samples effectively and assign the appropriate diagnostic category.21,22
Telecytopathology as a solution. Following the trend of telepathology that made it possible to obtain pathology consultations remotely, telecytopathology has now become a useful way to have cytopathologists present virtually during the ROSE procedure.23-27 A cytotechnologist can now send real-time video images of a case from distant sites to the cytopathologist’s office for the adequacy assessment, preliminary diagnosis, and optimal triage of the biopsy sample. This approach provides sufficient justification to bill for this service as a solution to the abovementioned reimbursement problem associated with ROSE.20

Fig. 2. FNA /ROSE mobile cart setup with microscope equipped with camera and MS Surface Pro for telecytopathology.
The images obtained from telecytopathology can be sent through static image transmission, live image streaming, and robotic live image streaming. Studies have shown that static image transmission is better suited to low-volume settings and highly skilled on-site cytology personnel. Live image streaming offers advantages over static image transmission as the process is more time efficient and allows the cytopathologist to review the whole slide. The limitation of the use of a robotic microscope for ROSE is the cost of the equipment, which is higher than for live image streaming.24-26
Regardless of the platform used for ROSE, validation is required. In our efforts to implement telecytopathology, we followed the CAP telepathology guidelines. When using the 60-slide validation study for whole slide imaging scans, which closely emulates the clinical environment, the concordance between WSI and glass slides approaches 95 percent.28 For our real-time video telecytopathology, we selected for our study 55 cases with 60 slides of cytologic preparation, i.e., FNA biopsy smears and core imprints.
During several sessions, one of our cytotechnologists initiated a live video feed over Zoom from our satellite Northside facility using a Nikon Eclipse Ci microscope on the FNA biopsy cart equipped with a microscope camera plugged into Microsoft Surface Pro (Figs. 1 and 2).
Zoom meeting software over the secure University of Florida intranet was used for sharing Surface Pro desktop, including audio communication. After each case was viewed, the participants were asked to evaluate each case separately. The results of this study showed an overall agreement of 96 percent in the assessment of adequacy among the participating cytopathologists, including the cytopathology fellow. The minor discrepancies during this validation process were mainly due to interpretive issues, based largely on the experience of cytopathologists.
We demonstrated that our approach in implementing telecytopathology was cost-effective: Our total equipment cost excluding the microscope was about $3,000. Currently, we are able to use telecytopathology during rapid off-site evaluation between our satellite locations and our cytopathology laboratory at the main hospital, and we are starting to bill for our services using CPT code 88172. Telecytopathology can also be used to provide real-time educational opportunities for cytopathology fellows and pathology residents.29,30
- Dudgeon LS, Patrick CV. A new method for the rapid microscopical diagnosis of tumours: with an account of 200 cases so examined. British J Surg. 1927;15(58):250–261.
- Mavec P. Cytologic diagnosis from tumor tissue using the “quick method” during operation. Acta Cytol. 1967;11(3):229–230.
- Scopa CD, Melachrinou M, Apessou D, Bonikos D. Tissue imprints in surgical pathology: a rapid intraoperative diagnostic aid. Diagn Cytopathol. 1990;6(1):5–8.
- Scucchi LF, Di Stefano D, Cosentino L, Vecchione A. Value of cytology as an adjunctive intraoperative diagnostic method. An audit of 2,250 consecutive cases. Acta Cytol. 1997;41(5):1489–1496.
- Naveed H, Abid M, Hashmi AA, et al. Diagnostic accuracy of touch imprint cytology for head and neck malignancies: a useful intra-operative tool in resource limited countries. BMC Clin Pathol. 2017;17:25.
- Ghandur-Mnaymneh L, Paz J. The use of touch preparations (tissue imprints) in the rapid intraoperative diagnosis of metastatic lymph node disease in cancer staging procedures. Cancer. 1985;56(2):339–344.
- Bakhshandeh M, Tutuncuoglu SO, Fischer G, Masood S. Use of imprint cytology for assessment of surgical margins in lumpectomy specimens of breast cancer patients. Diagn Cytopathol. 2007;35(10):656–659.
- Masood S. Immunocytochemical localization of estrogen and progesterone receptors in imprint preparations of breast carcinomas. Cancer. 1992;70(8):2109–2114.
- Masood S. Use of monoclonal antibody for assessment of estrogen and progesterone receptors in malignant effusions. Diagn Cytopathol. 1992;8(2):161–166.
- Masood S, Feng D, Tutuncuoglu O, et al. Diagnostic value of imprint cytology during image-guided core biopsy in improving breast health care. Ann Clin Lab Sci. 2011;41(1):8–13.
- Kubik MJ, Bovbel A, Goli H, Saremian J, Siddiqi A, Masood S. Diagnostic value and accuracy of imprint cytology evaluation during image-guided core needle biopsies: review of our experience at a large academic center. Diagn Cytopathol. 2015;43(10):773–779.
- Schmidt RL, Witt BL, Lopez-Calderon LE, Layfield LJ. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300–308.
- Anila KR, Nayak N, Venugopal M, Jayasree K. Role of rapid on-site evaluation in CT-guided fine needle aspiration cytology of lung nodules. J Cytol. 2018;35(4):229–232.
- Torous VF, Lopez SH, Xu C, Sweeney BJ, Pitman MB. Performance of rapid on-site evaluation in breast fine-needle aspiration biopsies: identifying areas of diagnostic challenge. Acta Cytol. 2022;66(1):1–13.
- Davenport RD. Rapid on-site evaluation of transbronchial aspirates. Chest. 1990;98(1):59–61.
- Paulose RR, Shee CD. Value of imprint cytology for ultrasound-guided transthoracic core biopsy. Eur Respir J. 2005;25(4):772.
- Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. 2013;121(9):518–524.
- Collins BT, Chen AC, Wang JF, Bernadt CT, Sanati S. Improved laboratory resource utilization and patient care with the use of rapid on-site evaluation for endobronchial ultrasound fine-needle aspiration biopsy. Cancer Cytopathol. 2013;121(10):544–551.
- Schmidt RL, Walker BS, Howard K, Layfield LJ, Adler DG. Rapid on-site evaluation reduces needle passes in endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: a risk-benefit analysis. Dig Dis Sci. 2013;58(11):3280–3286.
- Dhillon I, Pitman MB, Demay RM, Archuletta P, Shidham VB. Compensation crisis related to the onsite adequacy evaluation during FNA procedures—urgent proactive input from cytopathology community is critical to establish appropriate reimbursement for CPT code 88172 (or its new counterpart if introduced in the future). Cytojournal. 2010;7:23.
- Meena N, Jeffus S, Massoll N, et al. Rapid onsite evaluation: a comparison of cytopathologist and pulmonologist performance. Cancer Cytopathol. 2016;124(4):279–284.
- Collins JA, Novak A, Ali SZ, Olson MT. Cytotechnologists and on-site evaluation of adequacy. Korean J Pathol. 2013;47(5):405–410.
- Alsharif M, Carlo-Demovich J, Massey C, et al. Telecytopathology for immediate evaluation of fine-needle aspiration specimens. Cancer Cytopathol. 2010;118(3):119–126.
- Collins BT. Telepathology in cytopathology: challenges and opportunities. Acta Cytol. 2013;57(3):221–232.
- Kerr SE, Bellizzi AM, Stelow EB, Frierson HF Jr, Policarpio-Nicolas MLC. Initial assessment of fine-needle aspiration specimens by telepathology: validation for use in pathology resident-faculty consultations. Am J Clin Pathol. 2008;130(3):409–413.
- Lin O, Rudomina D, Feratovic R, Sirintrapun SJ. Rapid on-site evaluation using telecytology: a major cancer center experience. Diagn Cytopathol. 2019;47(1):15–19.
- Hudson JB, Murray BA, Guiney M, Chen AC, Collins BT, Wang JF. Telecytology for EBUS-FNA immediate adequacy assessment: implementation experience at a large academic medical center. J Am Soc Cytopathol. 2014;3(3):137–141.
- Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722.
- Lin O. Telecytology for rapid on-site evaluation: current status. J Am Soc Cytopathol. 2018;7:1–6.
- Farahani N, Parwani A, Pantanowitz L. Whole side imaging in pathology: advantage, limitations, and emerging perspectives. Pathology and Laboratory Medicine International. 2015;7:23–33.
Dr. Masood is professor and chair, Department of Pathology and Laboratory Medicine, and Dr. Siddiqi is associate professor and pathology residency program director—both at the University of Florida, Jacksonville. Dr. Masood is a member of the CAP Cytopathology Committee.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
