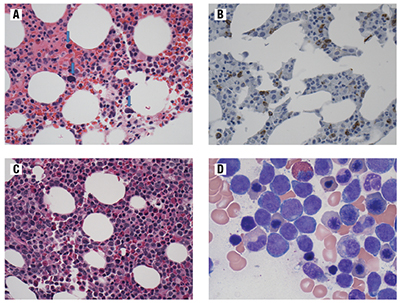
Fig. 1. Representative bone marrow morphological findings. (A) Bone marrow from the patient (same specimen as the CGAT) showing a hypocellular marrow with relative erythroid hyperplasia and dysplastic megakaryocytes (blue arrows). Stain: H&E, image taken with 40× objective. (B) Bone marrow from the patient (same specimen as the CGAT) showing a hypocellular marrow with increased scattered blasts (cells with brown pigment staining). Stain: CD34 immunohistochemistry, image taken with 40× objective. (C) Bone marrow core from the patient four months after CGAT study showing cnLOH. The marrow was hypercellular with increased immature mononuclear cells, which are the blasts. H&E-stained section, image taken with 40× objective. (D) Bone marrow aspirate from the patient four months after CGAT study showing cnLOH. This aspirate shows dysplastic features such as irregular nuclear contours and nuclear buds in the erythroid precursors with increased blasts (approximately 15 percent overall) in the marrow. Stained with Wright Giemsa, image taken with 100× objective.
The World Health Organization currently classifies tumors of hematopoietic and lymphoid tissue without reference to cnLOH results.<sup>1,3</sup> Although the mechanism leading to cnLOH has been postulated,<sup>4</sup> conclusions have yet to be drawn concerning patients with MDS and the association with cnLOH. In patients with AML, the presence of cnLOH is associated with a higher risk of disease recurrence and poorer patient outcomes.<sup>2</sup> This is a significant finding for patients with AML, yet we found an insufficient amount of literature outlining a comparable conclusion in patients with MDS. Because cnLOH can be detected only by CGAT (or SNP array) and not conventional cytogenetics or FISH, the current standard workup may underdiagnose some MDS patients. This case highlights the importance of CGAT findings of cnLOH and the need for future studies.
Conclusion. This case study raises the question whether the progression to high-grade MDS could have been avoided for this patient if therapy had been initiated in March 2014 considering the cnLOH as diagnostic evidence for his disease. The intriguing findings from this patient warrant the World Health Organization’s consideration of cnLOH as part of the diagnostic criteria similar to other cytogenetic abnormalities. The literature currently available for associations of cnLOH as a sole abnormality with MDS is limited, but the detection of cnLOH has proved valuable in the diagnosis of subtle disease.<sup>2,4</sup> A comprehensive evaluation including the CGAT findings of cnLOH can help providers classify disease types and prompt diagnosis and initial therapy.
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.
- Gronseth CM, McElhone SE, Storer BE, et al. Prognostic significance of acquired copy-neutral loss of heterozygosity in acute myeloid leukemia. Cancer. 2015;121(17):2900–2908.
- Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–87.
- O’Keefe C, McDevitt MA, Maciejewski JP. Copy neutral loss of heterozygosity: a novel chromosomal lesion in myeloid malignancies. Blood. 2010;115(14):2731–2739.
Christine Gronseth is a cytogenetic technologist and Scott McElhone is a molecular technologist, Department of Cytogenetics, Seattle Cancer Care Alliance. Dr. Yeung is an assistant professor and Dr. Fang is a professor, Department of Pathology, University of Washington Medical Center, Seattle. Dr. Scott is an associate professor, UWMC Department of Medicine. Dr. Yeung is an assistant member, Dr. Scott is an associate member, and Dr. Fang is a member of the Clinical Research Division, Fred Hutchinson Cancer Research Center.
[hr]Test yourself
Here are three questions taken from the case report.
Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. The 2008 WHO guidelines recognize which abnormalities as criteria for diagnosis of MDS? Cytopenia of undetermined origin in the blood and
a) >five percent blasts in the bone marrow.
b) <10 percent blasts in the bone marrow if unequivocal dysplasia is present and cnLOH.
c) <10 percent blasts in the bone marrow if unequivocal dysplasia is present and monosomy 5.
d) <10 percent blasts in the bone marrow if unequivocal dysplasia is present and t(2;11)(p21;q23).
e) Answers A, C, and D.
f) All of the above.
2. At a point in this patient’s disease progression, he demonstrated:
a) A FISH abnormality of monosomy 5.
b) Higher than 10 percent abnormal cells by flow cytometry.
c) A cytogenetic abnormality of del(7q).
d) An abnormality detected by CGAT (chromosome genomic array testing) at higher than 40 percent.
e) A copy number aberration by CGAT (chromosome genomic array testing).
3. Which is true regarding copy neutral loss of heterozygosity (cnLOH)?
a) It is detectable by conventional cytogenetics and FISH.
b) The mechanism leading to cnLOH has been reviewed.
c) It is associated with a higher risk of disease recurrence in patients with acute myeloid leukemia.
d) It is detectable by molecular techniques such as PCR-based analyses and hybridization-based CGAT (chromosome genomic array testing).
e) Answers B, C, and D.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
