
Dr. George
Hematologists and family practitioners will often ask when flow cytometry should be performed on peripheral blood samples in adults. One study (Andrews JM, et al. Leuk Lymphoma. 2008;49:1731–1737) reviewed flow results of patients age 50 and older who had absolute lymphocytic counts ≥ 4 × 109/L. These cases—as so often happens—had already been called suspicious for a lymphoproliferative disorder after smear review by a pathologist. The researchers found that if the absolute lymphocytic count is > 6.7 × 109/L, or the patient’s age was 67 or older, “This had a sensitivity of 95 percent and a specificity of 76 percent in predicting an abnormal phenotype by flow cytometry.”
There’s nothing magical about the number 67, by the way. “It’s just fortuitous that the 67 appears in both,” said Dr. George. In general, older patients and higher lymphocyte counts are more likely to predict an abnormal flow phenotype.
Granulocytic (either neutrophilia, basophilia, or eosinophilia) and monocytic leukocytosis present challenges of their own. “Mostly what we’re seeing are reactive neutrophilias,” also known as neutrophilic leukemoid reactions. The problem, at its essence, is production of too many neutrophils. “You’re demarginating them,” said Dr. George. “Or you have decreased egress of neutrophils from the peripheral circulation to the tissues.” The overwhelming cause is infections, though it can also be seen with inflammation, drugs, malignancy, hemorrhage, smoking, or any strong stimulus to the bone marrow.
Fig.5. Bordetella pertussis
Common clues pointing to reactive neutrophilia is a lower white blood cell count (50 × 109/L), pronounced left shift, basophilia or eosinophilia, dacrocytes (i.e. teardrop-shaped RBCs), or dysplasia.
When it comes to monocytosis, most will be reactive, Dr. George said. Reactive monocytosis can be seen with malignancy, such as gastric carcinomas, multiple myelomas, and lymphomas; chronic infections; autoimmune disorders; after splenectomy; and especially in regenerating marrows after either chemotherapy or transplant. “Remember,” she told her AACC audience, “the monocytes come back first, before the granulocytes.”
It can be challenging to distinguish between a promonocyte and an abnormal mature monocyte. That’s when it’s important to bring up a differential diagnosis: acute myeloid leukemia (AML), chronic myelomonocytic leukemia, myelodysplastic syndrome/myeloproliferative neoplasm. All of these diagnoses require a bone marrow examination, said Dr. George—despite what she was once asked by an outside hematologist sending a case to her reference laboratory. “He said, ‘Can’t you tell by just flow?’ And I said, ‘We really can’t. I need to know what’s going on in the bone marrow to make sure that this is not acute leukemia in this patient.’”
Eosinophilia is defined as a prolonged eosinophil count: >1.5 × 109/L for at least six months. In the algorithm Dr. George presented, the first step is for clinicians to screen for the reactive causes of eosinophilia, a list that can go “on and on,” Dr. George said. Once those are screened out, clinicians need to examine the peripheral blood and bone marrow—at which point they’ll likely order flow cytometry and T-cell clonality studies to look for an abnormal T-cell clone that could be prompting eosinophilia, or for another malignancy, such as Hodgkin’s lymphoma, a T-cell lymphoma, certain types of acute lymphoblastic leukemia, or a clonal eosinophilia due to chronic myelogenous leukemia or certain types of AML. Once those have been excluded, it’s appropriate to assess for tyrosine kinase mutations. “We’re looking for myeloproliferative neoplasms with eosinophilia and either platelet-derived growth factor receptor alpha (PDGFRA), -beta (PDGFRB), or FGFR1.
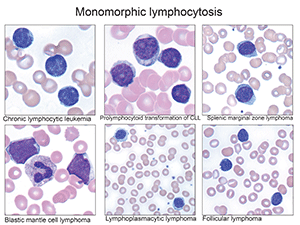
Fig. 6.
With PDGFRB and FGFR1, said Dr. George, “You’ve got cytogenetic abnormalities that you’re going to see by classical karyotyping. So you’ve got to do the karyotyping.” But because PDGFRA is cytogenetically cryptic, FISH or PCR will need to be done to look for PDGFRA mutations or the so-called CHIC2 deletion. If those are negative, and if there’s clonality or more than one percent blasts in blood or more than five percent in bone marrow, “you can call it chronic eosinophilic leukemia.” Absent all these, then it’s a case of hypereosinophilic syndrome.
It’s important to identify PDGFRA, PDGFRB, or FGFR1 “because alpha and beta respond to very small doses of imatinib,” Dr. George explained. “Essentially, you can cure these patients.” And with FGFR1, researchers are looking at alternative small molecule inhibitors.
Basophilia is relatively straightforward. “Overwhelmingly, if you have a true basophilia on a peripheral blood smear, you’re dealing with something neoplastic,” usually chronic myelogenous leukemia or another myeloproliferative neoplasm. Automated hematology instruments aren’t good at identifying basophilia, Dr. George cautioned. “This is something where you really do have to scan the slide with some modality.” And in general, because there’s an overwhelming association of neoplasia with basophilia, bone marrows are a must.
Regarding thrombocytosis, Dr. George noted that acquired causes are much more common than congenital ones. The latter are usually due to gain of function mutations in thrombopoietin or in MPL (a thrombopoietin receptor). Acquired causes can be primary or secondary, with the latter much more common—reactive thrombocytosis represents 85 percent of cases. Platelet counts can reach >1 million/µL (normal platelet count is approximately 150,000 to 450,000/µL).
Reactive thrombocytosis can be transient or sustained. In the former, clues include acute blood loss, so-called rebound from thrombocytopenia, acute infection or inflammation, or response to exercise. Sustained reactive thrombocytopenia, on the other hand, can be seen in cases of iron deficiency, hemolytic anemia, and asplenia. It can occur—lasting up to several months—after splenectomy. It can also be seen in cancer, chronic inflammation or infectious diseases, and even in drug reactions.
An underlying systemic disease is much more common in reactive thrombocytosis, while ischemia, thrombosis, and bleeding complications are more characteristic of clonal thrombocytosis. “That’s why you have to identify these patients,” she said. Peripheral smears can be helpful—but only up to a point. “Generally, by the time you’re thinking about a clonal thrombocytosis you’re getting bone marrows.”
Dr. George ended her journey not at Mytilene, but by talking about erythrocytosis. Although it’s defined as a more than two standard deviation increase from age-, sex-, and race-adjusted norms in both the hematocrit and hemoglobin, “You know that hemoglobin and hematocrit are affected by plasma volume.” That means pathologists will, like many a Shakespeare character, need to distinguish between competing identities. Is it an apparent erythrocytosis or the true erythrocytosis? Apparent is due to a decrease in plasma volume, which gives the appearance of an increased RBC content. This occurs when a patient is dehydrated or when there’s a chronically contracted plasma volume—it’s not a true increase in RBC mass.
“The way we think about this now is clonal erythrocytosis versus nonclonal, where the clonal overwhelmingly have a JAK2, either V617F mutation or a JAK2 exon 12 mutation,” Dr. George said. “And nonclonals do not.”
Ideally, clinicians will know if they are dealing with apparent erythrocytosis, “so hopefully, you’re just dealing with the true erythrocytosis.” It’s typical to start with JAK2 testing, reflexing to JAK2 exon 12 mutation testing if the JAK2 V617F is negative. In more than 95 percent of cases of polycythemia vera, JAK2 test results will be positive. In the remaining cases, erythropoietin testing should be done. “If it’s normal or increased EPO, then you’re dealing with an acquired erythrocytosis.”
Primary erythrocytosis is, typically, polycythemia vera. On a practical level—WHO classification be damned—clinicians look for an elevated hemoglobin, Dr. George said. If it’s normal, but they still suspect polycythemia vera, then they look for causes of a masked erythrocytosis, such as a concurrent iron deficiency anemia. They’ll look for one of the JAK2 mutations. “And they’ll look for a low or sometimes a normal EPO level.”
Bone marrow testing, in these cases, is conspicuous by its absence, Dr. George said. “I think we all are seeing this—for patients with suspected polycythemia vera, a bone marrow is generally not being performed until very late in the stage, where the patient then progresses to cytopenias and is developing post-polycythemic myelofibrosis.”
[hr]A few nuggets of advice
Dr. George offered these additional tips as part of her AACC presentation:
- “It’s key that you have a properly prepared blood smear to determine the etiology of anemia, cytopenias, and leukocytosis, whether you’re looking at it manually or with one of these image analysis systems. For isolated anemias, CBC parameters and reticulocyte count can help you determine a differential diagnosis. And you can use the peripheral blood smear and red cell morphology abnormalities to refine your differential diagnosis and determine what follow-up testing you’ll do.”
- “It’s important to exclude pseudothrombocytopenia and pseudoneutropenia before you undertake additional workups. And a peripheral blood smear review for cytopenias is often useful to detect clinically significant disorders, narrow diagnostic considerations, and focus your additional workup.”
- “Infections can result in cytopenias. And some organisms can be identified on the peripheral smear review.”
- “In patients with cytopenias, abnormal cells may be infrequent, but they can have significant impact on diagnosis and further workup. It’s important to rule out reactive causes first before jumping to the diagnosis of a malignant neoplasm. And it still remains true: a pleomorphic lymphocytosis favors a reactive lymphocytosis. But a monomorphic lymphocytosis should trigger additional ancillary studies in the correct setting.”
- “Neutrophilic leukemoid reactions are much more common than myeloid malignancies. Clues that you’re dealing with one of these leukemoid reactions include activated neutrophils and lower white cell counts. But if you are suspicious for a myeloid malignancy, then you need to perform a bone marrow examination as well as additional ancillary testing, which can include flow if blasts are elevated.”
Karen Titus is CAP TODAY contributing editor and co-managing editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
