Amy Carpenter Aquino
December 2020—As ongoing studies reveal the merits of emicizumab for hemophilia A patients—fewer bleeding episodes, longer duration between treatments—laboratories need to be alert to the drug’s effect on coagulation testing.
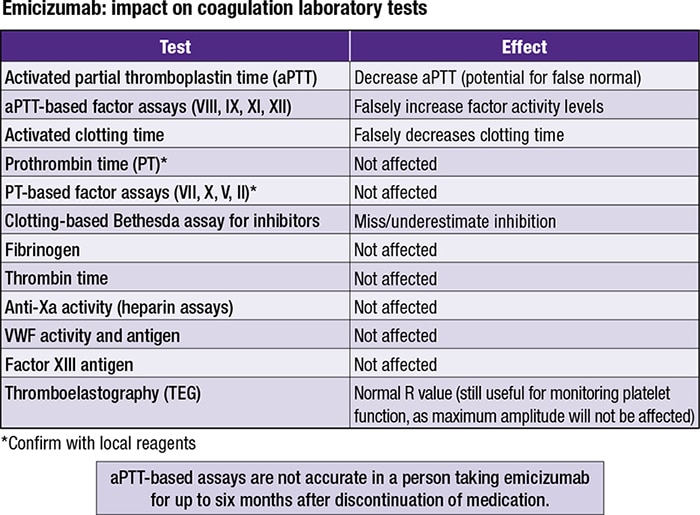
“If you take anything away from this presentation,” said Andrew J. Goodwin, MD, speaking in October in a CAP20 session, “aPTT assays in patients on emicizumab are not going to be accurate. And the effect of the drug on our aPTT-based assays can last up to six months following discontinuation of that medication.”
“It presents an interference, which is a real challenge,” said Dr. Goodwin, clinical pathology division chief and CLIA director, University of Vermont Medical Center. His co-presenter was David Unold, MD, associate medical director, transfusion services, University of California Davis Medical Center.
Dr. Unold reported the results of recent HAVEN studies of emicizumab (Hemlibra, Genentech), a bispecific factor IXa and factor X-directed antibody that mimics the activity of factor VIII. Emicizumab is unlike factor VIII replacement therapies because “it actually binds both the precursor—the zymogen—as well as the active forms of factors IX and X,” he said. It binds only human forms of factor IX and X, which is significant for laboratory testing.

Dr. Unold
“The nice thing about it, even though the schedule can vary based on the different concentrations available, is you can give the drug as few times as every four weeks,” Dr. Unold said. The half-life of the drug is 30 days (Müller J, et al. Thromb Haemost. 2019;119[9]:1384–1393).
The most recent trial of emicizumab—HAVEN 4—studied hemophilia A patients age 12 and older, with and without inhibitors. “Essentially, they wanted to look at this increased duration between injections,” Dr. Unold said. The trial found an annualized rate of treated bleeds of 2.4, which while slightly higher than the rates of 1.5 and 1.3 found in the earlier HAVEN 3 trial, still demonstrated benefits with a longer duration between injections. “And no thrombotic events or antidrug antibodies with neutralizing potential were identified,” he said (Pipe SW, et al. Lancet Haematol. 2019;6[6]:e295–e305).
Congenital hemophilia A, a genetic deficiency of the procoagulant factor VIII, is a disease negatively impacting secondary hemostasis, and in this disorder the PT would be normal, the aPTT would be prolonged, and the 50:50 mixing studies (incubated or nonincubated) “would correct into the normal range or to within whatever criteria are often used by the laboratory,” Dr. Goodwin said. And the fibrinogen and PFA-100 results would be normal.“In a patient with lupus coagulant—an acquired thrombotic disorder—depending on the reagents, the aPTT can also be prolonged,” Dr. Goodwin said. “But the difference is that the 50:50 mix fails to correct. So the 50:50 mix has some very good discriminating information with the results that can be used if you’re a reference laboratory or a laboratory that doesn’t have any history or information. A 50:50 mix is always a very good next step when you have prolonged screening coagulation tests.”
The fibrinogen and PFA-100 would be expected to be normal for a lupus coagulant patient, he added.
In patients with primary hemostatic disorders, such as von Willebrand disease, the PT and the aPTT can be normal. The aPTT can also be prolonged depending on the severity of the disease and the type of von Willebrand the patient has, Dr. Goodwin said.
Since the PT, aPTT, 50:50 mix, and fibrinogen test results can look similar to those of a patient with hemophilia, “and certainly in a patient with von Willebrand disease, we would have to do von Willebrand studies as well.”
In patients with fibron clot cross-linking or fibrinolytic defects that lead to a hemostatic disorder, the screening tests have limited sensitivity, Dr. Goodwin said. “They are not designed to pick up a patient, for example, with factor XIII deficiency. So in a patient who has delayed or significant bleeding following surgery—usually a few days later, when their screening coagulation tests are all normal—it’s time to start thinking about fibrinolytic defects.”
For specialized coagulation testing for hemophilia, “there are two main flavors of factor VIII activity monitoring”—aPTT-based clotting and chromogenic substrate assays—and there is variability in the values based on the methodology and the activator, Dr. Goodwin said.
“You can have normal aPTT results in a patient with hemophilia, particularly mild hemophilia. And in a male patient with a presentation or bleeding disorder that fits to a secondary hemostatic disorder and has concern for hemophilia A, if the clot-based activity is normal, it is recommended that you consider the phenomenon called discrepant hemophilia and follow up testing with a chromogenic FVIII activity assay.”
The chromogenic substrate assays are “more sensitive to certain mutations that are encountered, and that helps us in patients who present again with a bleeding disorder for whom we have a strong suspicion but a normal clot-based factor VIII activity,” he said. The chromogenic assay will have increased sensitivity and can show a lower factor VIII activity compared with the clot-based assay.
A paper published earlier this year showed that in some scenarios “the difference between the clot-based activity and the chromogenic activity could be on the order of two to four to even six times’ difference,” Dr. Goodwin said, with clot-based activity results being higher compared with chromogenic activity results (Al-Huniti A, et al. Am J Clin Pathol. 2020;154[1]:78–87).
Adamkewicz JI, Chen DC, Paz-Priel I. Effects and interferences of emicizumab, a humanised bispecific antibody mimicking activated factor VIII cofactor function, on coagulation assays. Thromb Haemost. 2019;119:1084–1093.
Müller J, Pekrul I, Pötzsch B, Berning B, Oldenburg J, Spannagl M. Laboratory monitoring in emicizumab-treated persons with hemophilia A. Thromb Haemost. 2019;119:1384–1393.
Jenkins PV, Bowyer A, Burgess C, et al. Laboratory coagulation tests and emicizumab treatment a United Kingdom Haemophilia Centre Doctors’ Organisation guideline. Haemophilia. 2020;26:151–155.
The impact of extended half-life factor replacement products on coagulation testing is noteworthy, Dr. Goodwin said.
“What’s important to understand when you look at the papers, often they’re going to present percent recovery of factor VIII in a patient receiving an extended half-life product. And the critical component you need to pay attention to is what’s the reagent, the assay type, and the activator being used. The activators behave differently in these extended half-life products” (Van den Bossche D, et al. Int J Lab Hematol. 2018;40[suppl 1]:21–29).
For example, Van den Bossche and coauthors found that an aPTT-SP reagent—a one-stage clotting assay with a silica activator—underestimated the percentage of factor VIII expected recovery of Afstyla (CSL Behring)—a single-chain, B-domain truncated, extended half-life medication—by about half (52 percent) compared with the chromogenic assay result. Using the correction factor of two, as indicated in the product insert, resulted in a percent factor recovery value of 104. “This is not a value of percent activity,” Dr. Goodwin noted.
“As a laboratory, we need to communicate with our clinicians and understand what particular medication a patient is being treated with for their hemophilia A so we are certain that the proper conversion factors are being used in a patient who’s having a clot-based assay,” Dr. Goodwin said.
In the Van den Bossche study, the chromogenic substrate assay showed better “percentage of FVIII:C expected recovery (%)” at normal levels, making it the manufacturer-preferred assay type for this medication.
However, when looking at low levels of factor VIII, for which the definitions can vary, “some of these assays perform quite well,” Dr. Goodwin said. The same aPTT-SP assay did recover about 100 percent of low levels of factor VIII (without using the correction), while the chromogenic assay overestimated the percent recovery at about 150 percent.
Dr. Goodwin explained that the impact of emicizumab, the first nonfactor replacement medication, on common coagulation assays is evident in the aPTT-based factor activity assay. He pointed to three recently published reviews (Adamkewicz JI, et al. Thromb Haemost. 2019;119[7]:1084–1093; Müller J, et al. Thromb Haemost. 2019;119[9]:1384–1393; and Jenkins PV, et al. Haemophilia. 2020;26[1]:151–155). CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
