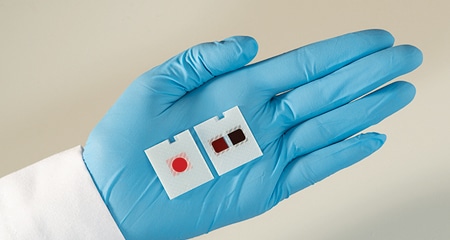
 June 2019—Ortho Clinical Diagnostics’ Vitros XT MicroSlides have been cleared by the FDA. The product features multitest technology that allows laboratories to run two tests simultaneously on one MicroSlide. Available test pairs include triglycerides and cholesterol, urea and creatinine, and glucose and calcium. By pairing tests so they can be run simultaneously, the microslides enable a higher test throughput without requiring additional or larger analyzers, the company reports. The miniaturized testing areas require less patient blood sample for each of the paired tests.
June 2019—Ortho Clinical Diagnostics’ Vitros XT MicroSlides have been cleared by the FDA. The product features multitest technology that allows laboratories to run two tests simultaneously on one MicroSlide. Available test pairs include triglycerides and cholesterol, urea and creatinine, and glucose and calcium. By pairing tests so they can be run simultaneously, the microslides enable a higher test throughput without requiring additional or larger analyzers, the company reports. The miniaturized testing areas require less patient blood sample for each of the paired tests.
The Vitros XT MicroSlides are for use on the company’s Vitros XT 7600 Integrated System.
Pages: 1 2
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
