For estrogen-positive cancers by IHC, the overall pathologic complete response to neoadjuvant chemotherapy was 10 percent. “However, when one applies MammaPrint and BluePrint to further stratify these patients, what you see are the pathologic complete response rates of two percent for luminal A, 5.6 percent for luminal B, and a very different pathologic complete response of 34 percent for the group that is genomically basal by BluePrint.” In this cohort, 13 percent of all the hormone-positive patients were reclassified as basal by BluePrint.
“With MammaPrint and BluePrint, we define luminal A as tumors that are low risk by MammaPrint and luminal in their BluePrint subtype, and we define luminal B as high risk by MammaPrint and luminal by BluePrint. The basal groups are nearly always high risk by MammaPrint. But by being basal through BluePrint, we are not detecting any hormonal signaling,” he said. When they limit their analysis to hormone-positive patients who are MammaPrint high-risk, the basal group makes up 29 percent.
When Dr. Audeh first saw this data, he suspected, as he said most clinicians would, that these ER-positive basal cancers were limited to the hormone-positive patients with the very low estrogen receptor expression by IHC. But that is not the case, he said, pointing to a scatterplot with all of the IHC levels for the ER-positive basal breast cancers. “While they do tend to cluster at the lower range of IHC, the range goes all the way up to 99 percent,” he said.
Progesterone receptor can also be positive, generally up to 50 percent. “So these receptors don’t definitively allow the identification of which patients are basal.” A comparison of pathologic complete response (pCR) rates between IHC-defined triple-negative cancers and BluePrint-defined ER-positive basal cancers reveals they are almost identical, compared with much lower pCR rates in luminal tumors by BluePrint, he said.
The longer term outcomes are also different. The five-year data were presented in San Antonio in 2020 in which the distant metastasis-free and overall survival outcomes were determined according to whether they had achieved a pCR (Whitworth P, et al. Cancer Res. 2021;81[suppl 4]:PD9-01). The basal tumors that achieved a pCR have an excellent outcome (93.7 percent probability of DMFS at five years), and, conversely, if they do not achieve a pCR, as with triple-negative breast cancers, the ER-positive basal cancers have a poor outcome (58.2 percent at five years). The probability of overall survival for ER-positive basal cancers that achieve a pCR is 82.1 percent, and for those that do not, 58.4 percent. “They behave in every way like triple-negative breast cancers,” he said of those that don’t achieve a pCR. “So we believe that BluePrint is essential to identifying these high-risk estrogen-receptor–positive breast cancers that are biologically and genomically in every way identical to triple-negative breast cancers, except for the fact that they express estrogen receptor protein, which appears to be nonfunctional.”
With the onset of the pandemic, Dr. Hicks and colleagues at the University of Rochester Medical Center began to be involved in the profiling of core needle biopsies. The recommendation made to health care systems was to delay elective care, including breast cancer surgery. In response, the Society of Surgical Oncology and the COVID-19 Pandemic Breast Cancer Consortium of the American Society of Breast Surgeons released guidelines suggesting that, in some cases, the use of core biopsies for genomic testing could be helpful in making decisions about surgical versus neoadjuvant treatment.
Dr. Hicks
“In dealing with a pandemic in our breast center,” Dr. Hicks said, “we’ve struggled with the following questions: Will genomics via core biopsy impact triaging patients who are essential versus nonessential for surgery under this current and difficult circumstance? And will early access to genomic test results lead to more efficient and effective breast cancer management decisions?”
The standard workflow in pathology at the URMC when evaluating core biopsies from newly diagnosed breast cancer patients would be to order ER, PR, HER2, and Ki-67 on the diagnostic core needle biopsy, and decisions on genomic profiling typically would remain postoperatively with tissue being sent from the resection specimen. “During the pandemic, we changed that workflow,” Dr. Hicks said. “We sent unstained slides for genomic profiling at the time the breast cancer biomarkers were ordered in hopes that genomic profile results would be back in time for the patient’s surgical consultation and treatment decisions.” This was part of a program called Preoperative Clinical Impact.
Between April and September 2020 they sent 211 specimens to Agendia for MammaPrint and BluePrint studies. Ten unstained slides were sent for genomic testing, and they were cut at the same time that the receptor testing was ordered. “We wanted to evaluate, first, the success rate of testing using these core biopsy tissues. What was the average turnaround time for results? Would results be available at the time of the surgical consult? And then what was the reclassification rate? How often were the genomics discordant with our clinical morphology and immunohistochemical results?” And finally, and probably the most challenging, he said: How often did this result influence treatment planning?
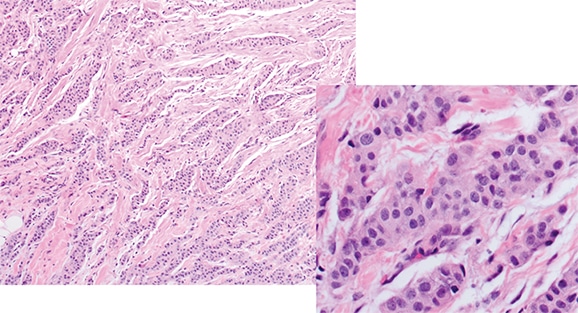
Fig. 2 [Images courtesy of David Hicks, MD.]
The reclassification rate—how often the results differed from their pathologic interpretation—was 32.3 percent (65/201). One ER-positive breast cancer came back with basal genomics (1/150). Of their triple-negative breast cancers, 16 percent came back as a luminal type (2/26 luminal A, 2/26 luminal B). Seventeen percent of the estrogen-receptor-negative, HER2-positive cancers came back as basal (1/6). Of the triple positives, 37 percent came back as luminal B (7/19) and five percent as luminal A (1/19). “And for what we would consider clinical high-risk tumors that were T2 and above and lymph node-positive, 39 percent came back as a luminal A subtype [26/67]. Of our clinically low-risk group, 62 percent came back as MammaPrint ultra-low [16/26].”
Dr. Hicks shared cases of these reclassifications that reveal how the genomics influenced their thinking. The first was that of a 74-year-old postmenopausal woman with no family history. She had an 8-mm mass on routine mammography, and a core needle biopsy was performed. “It’s an invasive carcinoma of an intermediate histologic grade. It has abundant eosinophilic cytoplasm, and a suggestion of some apocrine features.” (Fig. 2).

Fig. 3
The immunohistochemical panel came back ER-negative, PR-negative, and HER2-negative, “so the response on the part of the clinicians would be aggressive and would elicit thoughts about neoadjuvant chemotherapy.” The genomics came back with a luminal A molecular subtype and a MammaPrint low-risk. Neoadjuvant chemotherapy would be unlikely to have a good pathological response, according to the report. “So a very different genomic profile compared with our pathology,” Dr. Hicks said. This elicited an immediate call from the oncologist who wanted an explanation.
The pathology did provide hints, Dr. Hicks said. “This did not look like a typical basal phenotype breast cancer. It was an intermediate histologic grade, and it had apocrine features. So we performed an androgen receptor test, and all of the tumor cells were strongly positive for androgen receptor [Fig. 3]. What this case represents is an example of a luminal androgen receptor subtype of breast cancer, an unusual subset of triple-negative breast cancers that behave very differently from the typical triple negative.”
The second case is that of a 72-year-old postmenopausal woman who had a palpable mass that was 3.0 cm and a family history (sister with breast cancer). A core needle biopsy was performed. She had an invasive carcinoma of intermediate histologic grade (Fig. 4).

Fig. 4
Her tumor showed fairly strong expression of estrogen receptor (Fig. 5). The progesterone receptor was negative, HER2 was negative, and the Ki-67 proliferative index was fairly high. “By immunohistochemical subtyping, I would consider this a luminal subtype of breast cancer, probably a luminal B.” The genomics indicated a basal type with a MammaPrint high-risk. “So this would be one of those ER-positive basal breast cancers you heard about from Dr. Audeh.” Unlike what they anticipated from the immunohistochemistry, he said, the response to neoadjuvant chemotherapy in a patient with these genomics would be much closer to what one would expect from basal-type breast cancer.
“So we found that this accelerated utility workflow worked very well.” The genomic profiling has been an important step, he said, in optimizing risk stratification and treatment selections.
“And the pathologist has a role in helping to interpret this information in the morphologic context for the patient’s cancer. The genomic testing did play an important role in our treatment planning during the pandemic, and it was frequently discussed at tumor board. And I would receive calls from surgeons and medical oncologists.”
The approach has great potential for clinical utility beyond the pandemic, he said. “And our breast center right now is in active discussions about how we want to use this going forward.”
Agendia collaborates on several research protocols, one of which is the I-SPY trial, a neoadjuvant therapy trial seeking biomarkers for prediction of response. They have elected in their study design to use MammaPrint as a genomic screen for their hormone-positive patients. They will only enroll high-risk MammaPrint patients when they’re seeking pathologic complete response rates as their endpoint. Data generated by I-SPY and presented at a 2018 AACR meeting shows that as the MammaPrint index becomes higher in the high-risk range, the likelihood of a pathologic complete response goes up, indicating there is an association between MammaPrint risk and chemosensitivity (van’t Veer L, et al. Eur J Cancer. 2018;103[suppl 1]:e15–e16).
Fig. 5
The most important finding is that the highest end of the MammaPrint high-risk range, known as High 2 or MP2, has clinical meaning. That was displayed in one of the I-SPY trials published last year, in which standard-of-care chemotherapy (paclitaxel) was compared to the experimental arm in which two targeted therapies were used—immune checkpoint inhibitor durvalumab and PARP inhibitor olaparib—in combination with chemotherapy (Pusztai L, et al. Cancer Res. 2020;80[16 suppl]:CT011). MammaPrint High 2 was found to predict IO/PARP response in ER-positive breast cancer. “And we believe this will become a helpful biomarker for identifying ER-positive patients who may benefit from immunotherapy,” Dr. Audeh said. As with the low-risk range in MammaPrint, he added, they now have additional information in the high-risk range.
“And now as we split MammaPrint high-risk into High 1 and High 2, we also have these intriguing findings from I-SPY showing that these High 2 or ultra-high-risk MammaPrint patients are not only the most chemosensitive patients, but also they appear to have more sensitivity to immune checkpoint inhibitors such as pembrolizumab as well as PARP inhibitors and possibly even carboplatin.”
That leaves, as a subject for future research, the rest of the MammaPrint high-risk patients who are in the High 1 range, patients who need more than chemotherapy and endocrine therapy. “We believe this may be the group that may most benefit from CDK 4/6 inhibitors subject to future research that Agendia is undertaking.” Agendia’s FLEX study—it uses whole transcriptome data to expand breast cancer knowledge—enrolls all early-stage breast cancer patients, on whom MammaPrint and BluePrint are performed. “In the same run, in the same platform, with the same tissue sample, we are also able to obtain entire transcriptome data on each of these patients.” As part of this IRB-approved patient consent to trial, they also obtain multiple clinical data points and annotate the database. This database is then made available to their investigators around the country for substudies analyzing the subgroups within breast cancer.
Sherrie Rice is editor of CAP TODAY. The full webinar, made possible by a special educational grant from Agendia, is at https://www.captodayonline.com/combining-pathology-mammaprint-and-blueprint-to-inform-diagnostic-workup-and-treatment-planning-for-er-patients/.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
