In APTT-based assays, emicizumab will result in a shortening, or normalization, of the APTT response, which in the one-stage factor VIII activity assay will lead to an artificial overestimation of the factor VIII activity level, as high as 500 percent or more. Emicizumab, even if present at very low concentrations, can thus give the appearance that a particular patient has either normal or even elevated circulating levels of factor VIII, Dr. Tiefenbacher says.
If laboratory professionals aren’t being educated and aren’t aware of these APTT-based assay interferences, he adds, they are at risk to report incorrect laboratory results, which could adversely affect patient care. For example, if the inhibitor level in a patient treated with emicizumab is monitored and reported using a standard APTT-based inhibitor assay, the assay will provide a false-negative result, which the treating physician could incorrectly interpret as the patient no longer having a measurable FVIII inhibitor titer, he says.
Genentech wanted to forestall this kind of false-negative by making an alternative assay available: the chromogenic two-stage inhibitor assay. “As far as chromogenic assays go,” he says, “there are currently five different reagents available in the U.S. Only two or three of these assays utilize bovine-based factor IX and factor X and have been demonstrated to be suitable for the measurement of factor VIII inhibitors in the presence of emicizumab. Should a laboratory use a chromogenic assay that utilizes human-based reagents, it also will be interfered with by emicizumab. Due to the specific design of the humanized bispecific antibody, it does not cross-react with bovine origin reagents, as the antibody was selected to be specific to human factor IX and X.”
When LabCorp receives hemophilia A patient samples for testing, the typical test order received is for a factor VIII activity and a factor VIII inhibitor. Dr. Tiefenbacher provides an example of how test results can go awry: “We had a patient who previously demonstrated with significantly elevated APTTs, factor VIII one-stage activities at around one percent or below, and with an increasing factor VIII inhibitor titer as high as 200 BU. Suddenly, a month later, this same patient presents with a normal APTT result—in fact, slightly below normal—and a corresponding factor VIII one-stage activity result of greater than 500 percent.
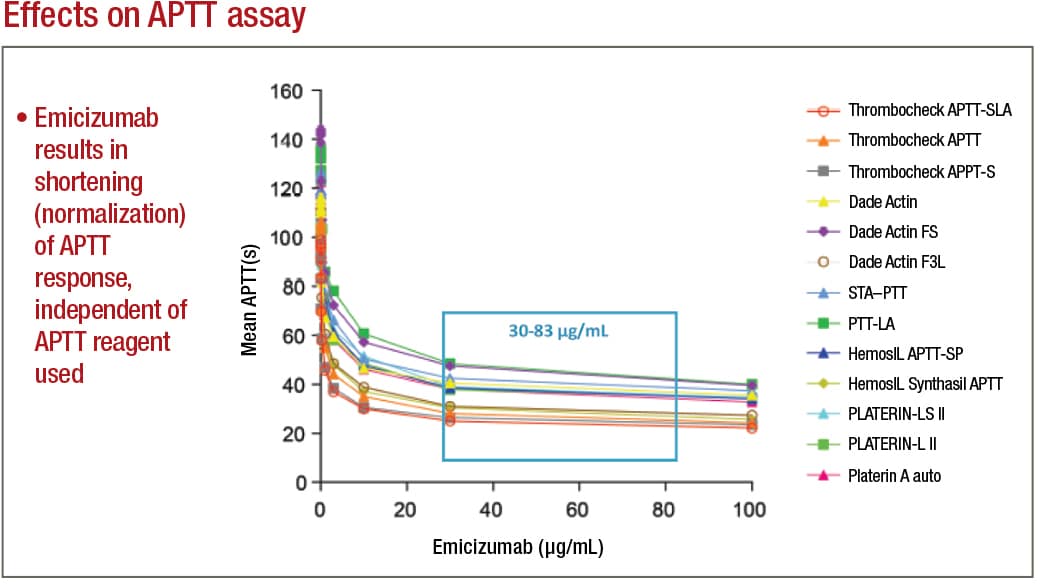
Adamkewicz, et al. Poster presentation at Hemostasis & Thrombosis Research Society, Scottsdale, Ariz., 2017; Courtesy of Genentech, Chugai, and LabCorp
“When the medical technologists brought these results to our attention, we suspected that the patient was switched from one of the traditional factor VIII replacement therapies to emicizumab. However, the treating physician appeared to have ordered the standard coagulation assays that are customary for patients receiving traditional factor VIII replacement therapy,” Dr. Tiefenbacher says. This same patient, when tested using a bovine reagent-based chromogenic assay, had nonmeasurable factor VIII activity. “We have since received several other patient samples from a variety of different hemophilia centers where the treating physician continued to order the standard lab testing associated with traditional factor VIII replacement therapy. Thus, there clearly is a need for additional education, both at the level of the ordering physician as well as at the laboratory level where the testing is performed,” he says.
Another potential future challenge for laboratories is that emicizumab is marketed as not requiring laboratory monitoring, he says. “However, one can come up with scenarios where laboratory measurement of emicizumab may be required, for example, when a patient requires surgery in an emergency-type setting. The treating clinician or surgeon and the laboratory will have to know that the patient is or has within the past six months been on emicizumab and that conventional APTT-based screening assays will provide inaccurate results.” It is Dr. Tiefenbacher’s understanding that some institutions are providing patients with bracelets that indicate the patient is on emicizumab therapy and that APTT-based assays cannot be used for monitoring, as is also stated on the drug label.
Since Colorado Coagulation is a reference laboratory, “we don’t always know what drugs the patient is taking,” Dr. Adcock says, “and the technologists in the lab have to exercise good judgment. When we see factor VIII activity that is extraordinary—for example, over 400 percent, where normal is up to 150 percent—then we question if the patient is on emicizumab, and we may reach out to the doctor or perform a chromogenic factor VIII activity assay.” As she explains, “If you want to obtain a semi-accurate activity of the emicizumab, you use the chromogenic human assay. To monitor factor VIII antibodies in patients on emicizumab, you use the bovine chromogenic factor VIII Bethesda inhibitor assay.”
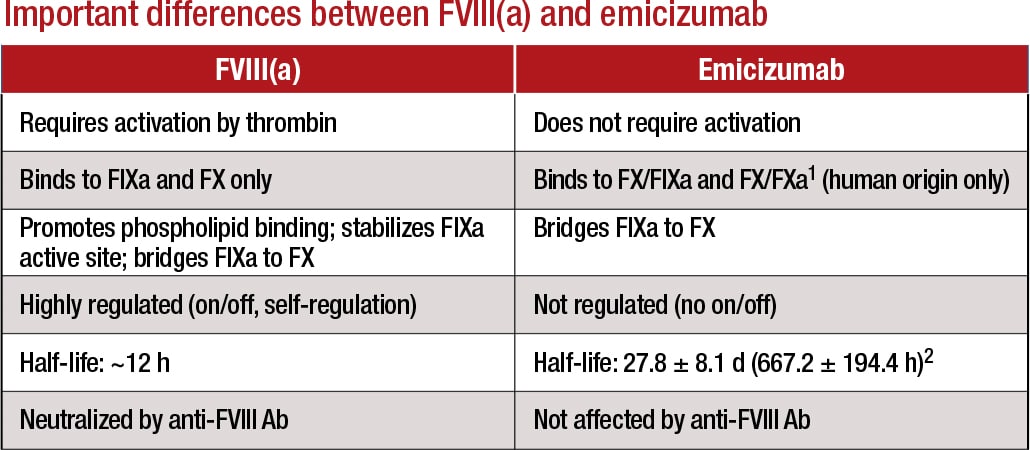
1Lenting, et al. Blood. 2017;130(23):2463–2468; 2Hemlibra FDA Prescribing Information, 11/2017 Courtesy of LabCorp
Dr. Tiefenbacher agrees: “We are a special coagulation lab, so we probably are more experienced in those areas, but it can take some detective work to determine if the observed interference in the patient sample on hand is due to emicizumab or some other type of drug or interference. Oftentimes we have little information provided for a particular sample other than the test order. This is where education and training of the involved parties becomes important.”
It’s also important to note, he says, that “until some type of neutralizer for emicizumab is developed, assay interference—at least in the APTT-based coagulation assays—should be anticipated for up to six months posttreatment. People need to be aware of this and use a bovine-based chromogenic assay when trying to measure inhibitors in these patients.”
Dr. Tiefenbacher makes five recommendations for coagulation laboratories addressing the testing needs of emicizumab patients: 1) avoid APTT-based coagulation assays such as APTT-based one-stage factor activity and factor VIII Bethesda inhibitor assays; 2) where possible, replace APTT-based assays with chromogenic or immunoassay-based coagulation tests; 3) use the chromogenic (bovine factor IXa and factor X) factor VIII Bethesda assay when measuring factor VIII inhibitors; 4) to measure emicizumab, use a chromogenic (human factor IXa and factor X) or dilute one-stage assay with emicizumab calibrator and controls; and 5) work closely with physician customers to provide education and training on emicizumab interference and the selection of the most appropriate tests.
Education will be crucial to avoiding serious adverse outcomes when laboratories test hemophilia patients, says Dr. Young, of Children’s Hospital Los Angeles. “The company that’s marketing the drug is working on educating the community. They are doing webinars and podcasts, conducting symposia at national meetings, and putting out flyers. But one problem will be, if patients are on the drug and somebody just decides, ‘I need a factor VIII level,’ without being aware of laboratory interference, they will misread the results and may make incorrect clinical decisions based on a test that should not have been done in the first place.”The hazard is more there than anywhere else, he notes. “But my ultimate nightmare scenario is when, let’s say, a patient who has hemophilia and is on this medication is traveling somewhere, has an auto accident and major trauma, and is perhaps unconscious. They get taken to a trauma center and the center says, ‘Oh my gosh, you have pelvic fractures. We need to do surgery right away.’ The surgeons measure APTT to determine if there is risk for bleeding, but this patient who actually has hemophilia will have normal APTT and will be okayed to have major surgery.”
That’s going to happen, he predicts. “The patient will have surgery, and while emicizumab will offer some level of coagulation protection from bleeding, if it’s a big surgery it’s probably not going to be enough, and there will be significant bleeding during the surgery.”
If the drug becomes as widely used as he expects, “labs need to ask themselves when they are receiving samples if they should alter their requisition form,” Dr. Young says, so that when factor VIII activity level or inhibitor level is ordered, there is a box to check if the patient is on emicizumab. “If the lab is aware whether the patient is on the drug or not, that can help them in ensuring a proper test is done and proper interpretation is done.”
A double check, where a laboratory receives a sample and there is an alert asking if the patient is on emicizumab, is another possible mechanism that might help. “I think that’s something the laboratory societies should be considering,” he says.
The vast majority of hemophilia patients are treated at one of the 140 hemophilia centers in the U.S., Dr. Young says. “But there are some patients outside those centers, and I worry about those patients even more, because doctors may see emicizumab as really useful, and the sales rep will recommend that they put patients on it, which is fine if it’s beneficial to their patients. But the doctors may not be hemophilia or coagulation experts, and that’s where we need to worry even more about misinterpretation of lab results.”
Still, he says, it should not be forgotten that emicizumab is a life-changing drug. One example he cites is the case of a 12 year old who was spending 30 percent of his time in a wheelchair, missing a lot of school, spending a lot of time in the hospital. “Now, since he was put on the drug two-and-a-half years ago, he hasn’t missed any school, he pretty much has no bleeding, he can do all kinds of physical activities, and the family got rid of the wheelchair a long time ago.”

Dr. Young
Dr. Young has also seen the same effect on younger patients. “There have been four and five year olds who were bleeding all the time, having a lot of pain, and their families have to do a lot of infusions. Now I have five kids younger than age 12 who have been on the clinical trial of emicizumab an average of two years and none of them has had a bleed at all. They’ve gone from 15 to 20 bleeds a year, pain, damage to joints, and multiple IV infusions to basically being a normal kid and not having to do any infusions other than weekly subcutaneous ones.”
Soon, non-inhibitor hemophilia patients may also benefit from emicizumab, says Genentech’s Dr. Levy, pointing to exciting clinical trial results for Hemlibra in people without factor VIII inhibitors. “Our HAVEN 3 study showed that Hemlibra prophylaxis resulted in a statistically significant and clinically meaningful reduction in treated bleeds compared to no prophylaxis. It also showed Hemlibra is the first medicine to significantly reduce bleeds compared to prior factor VIII prophylaxis, the current standard-of-care treatment for people without factor VIII inhibitors, as demonstrated by a statistically significant reduction in treated bleeds in an intrapatient comparison” (Mahlangu J, et al. N Engl J Med. 2018;379[9]:811–822).
Based on the data from the HAVEN 3 trial, Genentech and Roche filed a supplemental biologics license application and were granted priority review for Hemlibra to treat adults and children with hemophilia A without factor VIII inhibitors. The FDA is expected to announce a decision on approval of this widened application in early October, Dr. Young says.
In the meantime, Genentech continues to address the issue of accurate test results by stressing education. “Education of patients and physicians is something that we take very seriously and will continue to do,” Dr. Levy says. “Separately, we are working to support the continued development of existing tests that may be of use to the hemophilia community, as well as new tests that may be of help to physicians in certain clinical scenarios.”
Anne Paxton is a writer and attorney in Seattle.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
