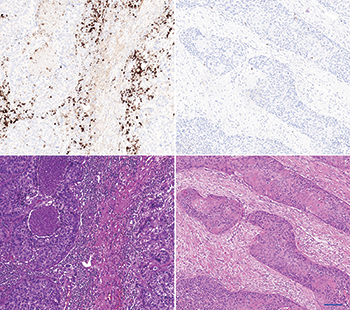
The left panel shows an “inflamed” carcinoma. The upper left shows a strong PD-L1 immune cell staining by SP142/ Ventana. The lower left shows the corresponding HE stain. The stroma is cell rich and contains a high density of immune cells. The right panel shows an “immune desert” carcinoma. The upper picture shows a negative PD-L1 immune cell staining by SP142/ Ventana. The lower right shows the corresponding HE stain. The stroma is fibrous and contains fibroblasts without immune cells. Scalebar is 100 µm.
Dr. Sholl discussed other potential biomarkers for predicting which patients might be helped by the PD-1 and PD-L1 inhibitors. “Some groups have described the correlation between the tumor mutational burden and response to immune checkpoint blockade,” she said. “And others have proposed a combination of tumor and immune cell profiling to define a ‘hot signature’ that may predict response to these drugs. As we all know, the best biomarker must be firmly grounded in science but must also be accessible to pathologists in diverse practice settings.”
Dr. Sholl asked Dr. Lathan in the plenary how things such as tumor mutation burden, “which actually correlate with a patient’s smoking history and potentially with KRAS mutations,” affect his decision to consider immunotherapy for a particular patient in the second or later line. Eighty-five percent of lung cancer patients have had tobacco exposure, Dr. Lathan noted, and 25 percent have a KRAS mutation. “I just don’t operationalize that,” he said, adding that he stores such information as “potentially useful in the future.”
Tumors that don’t respond to a PD-1 inhibitor don’t seem to have many T cells when the pathologist looks at them, Dr. Freeman says. “The idea is that the PD-1 drug doesn’t start the immune response from nothing but frees a smoldering but inhibited immune response to be effective. It’s allowing the T cells already in the tumor to be more active and attack the tumor,” he explains. “An idea people are focusing on now is how do you get the tumor to be infiltrated with T cells that can attack it?”
In a separate Roche-sponsored talk at CAP16 on PD-1 inhibitors and the tumor microenvironment, Mark Kockx, MD, PhD, a pathologist and medical director at HistoGenex in Belgium and Naperville, Ill., showed images (at left) that underscore how important it is to see if a tumor has ongoing inflammation or if it is noninflamed, which is also called an immune desert. Many factors lead to this immune desert condition, he said in an interview with CAP TODAY. “Cell types and moieties within the stroma of the tumor microenvironment,” such as cancer-associated fibroblasts, can induce substantial immunosuppression.
Dr. Kockx postulates that the resistance biomarker for PD-L1 and PD-1 checkpoint drugs may be the presence of cancer-associated fibroblasts in the tumor, in addition to an immune desert and an absence of PD-L1 expression. “The pathologist is necessary to manage this nuanced complexity,” he says.
At the moment, looking for inflammation in and around the tumor is how groups of patients tested for PD-L1 expression are potentially “bucketed” in pathology, agrees Roger Dansey, MBBCh, senior vice president of global clinical development oncology, Merck Research Laboratories. “I think our focus is not so much on the histologic appearance,” he tells CAP TODAY, “but more at looking at changes in genes so we can group patients using RNA expression analysis into those that represent the inflamed responders, inflamed nonresponders, and the noninflamed nonresponders.” (He notes that the latter would be equivalent to the immune desert.) “One of the challenges in introducing new testing is that you want something that’s highly reproducible—and gene expression profiling could be an alternative option to IHC,” Dr. Dansey says.

Dr. Dansey
“Biological pathways,” says Dr. Kockx, “also contribute to immunosuppression—for instance, the angiogenic pathway where the integral VEGF molecule itself has an immunosuppressive action. These elucidations provide a rationale to combine anti-angiogenic drugs like Avastin with checkpoint receptor inhibitors, and therefore convert an immune desert to an immune active environment.”
Radiation or conventional chemotherapy might also do the trick. “Inducing cancer cell death,” Dr. Kockx says, “can increase immunogenicity by exposing tumor neoantigens to the immune system. This in turn triggers response from the immune cells, which we have observed in samples following chemotherapy or radiation—an increased influx of immune cells.”
There’s another potential tactic. Merck and partner Amgen are conducting clinical trials in which the researchers inject an oncolytic virus directly into tumors. Says Dr. Dansey: “The virus is genetically modified to express granulocyte-macrophage colony-stimulating factor, or GM-CSF. The combination of the virus that causes lysis of tumor cells plus the local release of GM-CSF which attracts macrophages and other cells into the tumor microenvironment—and the addition of Keytruda given systemically—is the same sort of concept as chemotherapy or radiation where you are causing injury in the tumor that’s allowing an inflammatory response to develop and then taking the brakes off the immune response with PD-L1 inhibition and looking for improved outcomes.”

Dr. Kockx
Dr. Kockx points to two other immunotherapy modalities: personalized vaccines and engineered T-cell therapy. The identification of cancer neoantigens, although still novel, will be important to identify candidates for either of these methods, in his view. (In T-cell therapy, T cells are removed from a person’s body, trained against the cancer antigen in the laboratory, and then reinfused into the person, he notes.)
The two modalities also offer combination strategies with PD-1 and PD-L1 inhibitors, which have been shown in melanoma, Dr. Kockx says. “This method still requires surveillance of the tumor microenvironment, since a tumor landscape containing immunosuppressive elements will mount formidable resistance against vaccines or engineered T cells.” By systematically interrogating the tumor microenvironment with a combination of morphology and molecular technologies, he says, “we can develop a treatment strategy to leverage the supportive effects of the tumor microenvironment while evading or eliminating the suppressive elements.”
“Now that PD-1 has opened the door,” Dana-Farber’s Dr. Freeman says, “people realize the immune system can successfully attack cancer. There is just an incredible amount of energy and enthusiasm going into scientific studies in academia and the pharmaceutical industry to find things that work with PD-1 to increase the success rate, to extend it to other tumor types in a combination to do better. It’s abundantly clear that there are combinations that work together to do even better.”
Recent approvals and the pipeline
Worldwide, Dr. Freeman says, there are 20 PD-1 or PD-L1 drugs in 803 clinical trials with 166,736 patient slots ongoing. “We will find out what works best, what’s safest, who it works for, how it works, and how to cure many cancers.”
[hr]
Karen Lusky is a writer in Brentwood, Tenn.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
