Karen Titus
September 2021—Rare wine? Delectable. Rara avis? Magnificent.
Rare blue-top collection tube? Uh oh.
For Richard Marlar, PhD, coming across a non-FDA-approved tube was an unhappy discovery. Dr. Marlar, medical director, coagulation laboratory, University of New Mexico Hospital, says his lab was among the first to encounter one of these rogue tubes, available for purchase on the internet and likely taking wing due to pandemic supply shortages.
When the tube arrived for testing, it quickly kindled concerns, says Dr. Marlar, also a part-time professor of pathology, UNM School of Medicine. “It’s a tube we had never seen before. It looks like it has a CE mark on it, and the Europeans don’t know anything about it. It has a label on it that suggests it’s FDA approved—but the FDA is not aware of it,” he says, adding that his lab has spoken with the agency.
It feels like a “CSI”-tinged moment in a venue that labs would prefer to keep drama-free. It also points to the ongoing need to keep a keen eye on what passes through coagulation laboratories. It’s not so much that the devil is in the details; rather, that’s where accurate results lie.
So important are these details that they’re the focus of a new set of recommendations from the International Council for Standardization in Haematology on collecting blood samples for coagulation testing (Kitchen S, et al. Int J Lab Hematol. 2021;43[4]:571–580). As the report notes, one third to three quarters of laboratory errors are linked to the preanalytical phase, with coagulation testing accounting for many of the woes.
The recommendations were last updated in 2008, says Dr. Marlar, who helped author the current document. While numerous individual publications on the topic have emerged in the last decade-plus, “We wanted this to be an evidence-based set” of recommendations, he says. A subsequent document, authored by largely the same group of experts, focuses on processing coagulation samples and is likely to be published by year’s end, if not before.
Collecting and preparing samples for coagulation testing is a surprisingly treacherous endeavor. “Chemistry, immunology, hematology—they’re much more forgiving than what we see in coagulation,” Dr. Marlar says.
The runway leading to the coag lab is long. Ground turbulence can occur anywhere along the way when personnel don’t follow strict protocols regarding fill volumes, matrix, length of time to processing, order of draw, tourniquet use, ordering systems, etc.
“The preanalytic phase is the hardest phase to control because it can occur in so many different regions of patient care,” says ICSH recommendations coauthor Dorothy Adcock, MD, former chief medical officer, Labcorp Diagnostics, and, before that, medical director, Colorado Coagulation.

Dr. Richard Marlar and Dr. Dorothy Adcock helped write the ICSH recommendations for the collection of blood samples for coagulation testing. “I’ve always been surprised, with coagulation testing in particular, at the impact some variable may have,” Dr. Adcock says.
The impact on medical decisions is real. After decades in the field, Dr. Adcock has seen plenty. But her encounters with the unexpected have yet to end. “I’ve always been surprised, with coagulation testing in particular, at the impact some variable may have.” The stories get told in various ways, each with its own particulars, but the theme is constant—pay attention to the fine print.
When she began her career, in the 1990s, “One of our really astute techs—and I love how much I’ve learned from our med techs—mentioned to me that when she got to the patients’ samples soon after collection, the heparin level was significantly higher than if she had the samples sit on the bench for a couple of hours.”
That observation led Drs. Adcock and Marlar to investigate and publish a study (Adcock D, et al. Blood Coagul Fibrinolysis. 1998;9[6]:463–470) about the release of platelet factor 4 in citrated blood post-collection and its neutralization of heparin. “Which is why, when you’re collecting a sample, for unfractionated heparin, you need to process it, centrifuge it, within an hour, and test it within four,” says Dr. Adcock.
Cue the chorus: “That was such a surprise to me,” she says. “But it had to be investigated. Because you just never know what the impact will be.”
That story is set nearly a quarter of a century ago, which practically qualifies as ancient history in medicine. But the lesson continues to reverberate—in coagulation testing, doing without thinking can lead to errors. That’s why, says Dr. Adcock, this most recent document is “a good reminder to review your blood collection processes, especially for coagulation samples, and make certain they’re in line with current guidance.”The recommendations are based on what has become a fairly robust literature, which is a welcome shift. For many years practice has been heavy on tradition. While that might work for, say, the Royal Family, it’s not a basis for high-quality coagulation testing. Or as Dr. Marlar puts it: “We found that that doesn’t necessarily work.” For example, one long-standing habit was to use a discard tube, which, as it turns out, mostly serves to waste patient blood and increase costs.
He and Dr. Adcock, along with others, have published studies on this, as the recommendations make clear (Adcock DM, et al. Laboratory Medicine. 1997;28[8]:530–533; Lippi G, et al. Blood Coagul Fibrinolysis. 2012;23[6]:572–573), and recommendation No. 5.3 dispenses with a general requirement to discard the first volume of blood collected, unless it’s collected via butterfly needles or indwelling catheters, or when the sample is used for platelet function studies.
Other studies have looked at the impact on laboratory values of underfilling tubes, “whether it’s by 50 percent, 80 percent, or what have you,” says Dr. Marlar. ”We’ve started doing research, and a lot of issues are emerging. We’re still working on it. We’re still learning.”
Collection tubes might come across as the steady, unsung gal pals of coagulation testing, but in reality, they can be quite the divas. Labs that ignore them do so at their patients’ peril. Tubes can vary slightly—between 3.1 and 3.2 percent—in their exact sodium citrate concentration. They can be made of siliconized glass or different types of plastic. The composition of their stoppers varies, too. As Dr. Adcock notes, some stoppers may leak magnesium, which has been shown to influence results. And not every vacuum is sufficient to draw the correct amount.
It has taken time for laboratories to recognize these differences. When the INR was being rolled out in the late 1980s, Dr. Adcock recalls, two concentrations of sodium citrate were in common use: 3.2 and 3.8 percent. While the big box containing the hundred evacuated tubes was labeled, she says, individual tubes were not. “Some laboratories were not even aware there was a difference between these two concentrations,” and test orders would be randomly placed for each type.
When she and Dr. Marlar published on this in 1997, she says, one major tube manufacturer stepped up and began indicating the citrate concentration on individual tubes. When reagent manufacturers determine the ISI, or International Sensitivity Index, for each reagent, she says, the overwhelming majority of them perform that testing in 3.2 percent citrate or its equivalent. Size may not matter, but concentration does.
The ICSH document reflects this hard-won knowledge. Recommendation No. 3.2 calls for citrated blood to be anticoagulated with 105–109 mmol/L (3.1–3.2 percent) trisodium citrate, barring other indications. The next recommendation, No. 3.3., recommends against using 129 mmol/L (3.8 percent) trisodium citrate.
One of the most common errors, Dr. Adcock says, is using the wrong matrix when submitting samples for hemostasis testing. “That can significantly impact results.” (See “Matrix effect.”)She describes encountering one case involving a 70-year-old woman, needing a hip replacement, with no previous history of bleeding. The APTT was prolonged and did not correct with a mixing study; it showed some prolongation with incubation, and a low—3.8 percent—factor VIII.
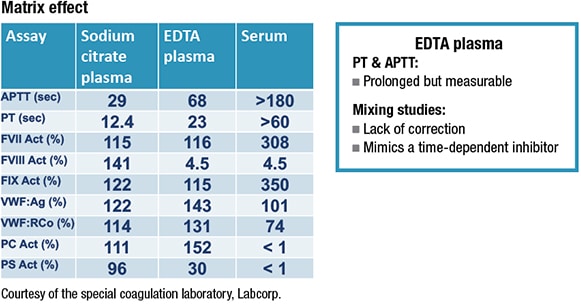 After her lab received the sample for confirmation of a factor VIII inhibitor, it ran a PT and PTT to check the sample integrity. Both were prolonged. “This pattern of results is very typical of EDTA but is not what you would expect to see with a factor VIII inhibitor,” says Dr. Adcock. “And EDTA as a sample matrix can perfectly mimic a factor VIII inhibitor.”
After her lab received the sample for confirmation of a factor VIII inhibitor, it ran a PT and PTT to check the sample integrity. Both were prolonged. “This pattern of results is very typical of EDTA but is not what you would expect to see with a factor VIII inhibitor,” says Dr. Adcock. “And EDTA as a sample matrix can perfectly mimic a factor VIII inhibitor.”
The ICSH authors note this “important and highly specific preanalytical problem,” explaining that citrated plasma can be contaminated with other additives such as EDTA or activated factors from serum, either through inadvertent cross-contamination between tubes during venipuncture, or by filling a coag tube with EDTA blood or serum.
Dr. Adcock also recalls the case of a medical technologist who had been on warfarin for six months, but four weeks after it had been discontinued, pre-surgery bloodwork indicated a prolonged PT (27 seconds) and PTT (42 seconds). “They asked, could this be some sort of prolonged warfarin effect?” Dr. Adcock says. “Well, no, it couldn’t.”
The technologist, concerned about the results, called Dr. Adcock. After learning that the phlebotomy was difficult and required multiple blue-top tubes, Dr. Adcock suspected the phlebotomist had combined two underfilled tubes. The excess sodium citrate would prolong the results. When the technologist was redrawn—“in our lab, under my own eyes,” Dr. Adcock says—the repeat PT and PTT were normal.
The recommendations are a tidy summary of the state of coagulation collection, bringing together the past, present, and future.“Many of the recommendations have been around for a long time,” Dr. Marlar says, but have yet to widely sink in.
“There are stubborn problems,” Dr. Adcock agrees. She recites the familiar stanzas: “Hemolysis is a big problem. Sample clotting is a big problem. Inappropriate blood to anticoagulant ratio is another problem.”
In some cases, the recommendations break fresh ground. “We’ve modified our ideas over time, and I think that happens with anything,” Dr. Marlar says. In the past, there was an absence of guidance on how long to apply a tourniquet. “Now we’re saying no longer than two minutes,” says Dr. Marlar, referring to recommendation No. 6.1. When the tourniquet is in place longer, it can affect test results, including hemostatic proteins stored in vascular endothelium.
That two-minute mark is not the goal, adds Dr. Adcock. “Really, it should be taken off as soon as you are able to begin collecting blood through an evacuated tube system.”
Laboratories also struggle with fill volumes. Some 5-mL tubes, for example, are designed to take only 3 mLs of blood, giving it the appearance of being just over half filled, says Dr. Marlar. “We occasionally find those tubes where someone says, ‘Oh, it’s not filled. I’m going to put more blood in it.’ And then they overfill it,” as if topping off a car’s gas tank.
Then there’s the order of draw, although Dr. Marlar says it appears the tide is turning on this particular problem. Recommendation No. 7.1 lists the ideal order for filling multiple tubes, starting with the blood culture tube and ending with so-called other tubes (trace elements, for example). This lowers the risk for cross-contamination, although the authors note that the current generation of evacuated blood collection tubes has nearly stamped out this problem.
Remember, says Dr. Adcock, collection devices—the preferred term, she says—should be viewed as an integrated system, comprising the holder, tube, and needle.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
