Amy Carpenter Aquino
February 2021—Eighteen months after introducing a next-generation sequencing assay to detect CMV antiviral resistance in the transplant population, Matthew Binnicker, PhD, D(ABMM), in an AMP presentation, shared a patient’s case and his laboratory’s broader experience.
The diagnostic advantages of the NGS assay are earlier detection of resistance and better management of cytomegalovirus in transplant patients, said Dr. Binnicker, director of clinical virology and vice chair of practice, Department of Laboratory Medicine and Pathology, Mayo Clinic, in the virtual AMP2020 presentation in November.
As the number of transplants rises in the United States, he said, “we’re going to see an increasing number of individuals who are on high levels of immunosuppressive medications, and this puts them at an increased risk of infectious diseases like CMV.”
Dr. Binnicker, who is also professor of laboratory medicine and pathology, discussed the Mayo Clinic case of a 67-year-old female patient with a history of polycystic kidney disease, who underwent a living, unrelated-donor renal transplant. Her serostatus was determined pre-transplantation to be a mismatch for CMV IgG: “The donor was seropositive, but the recipient was seronegative.” The patient’s Epstein-Barr virus serostatus was donor-positive, recipient-positive.
The patient presented five months post-surgery with elevated liver enzymes and described two days of diarrhea leading up to her presentation. Because of these clinical manifestations and the patient’s serostatus pre-surgery, the team ordered a CMV viral load on a plasma sample, which was reported as elevated at 130,000 IU/mL—“definitely a result that would be concerning, along with these clinical manifestations, for possibly a CMV disease in the post-transplant setting,” Dr. Binnicker said.
The patient was started on ganciclovir, and a test performed the following week found an elevated CMV viral load of more than 1 million IU/mL. “It’s not too concerning to see a viral load increase even after an antiviral has started. We need to wait at least a few weeks before we begin thinking about resistance or changing management,” Dr. Binnicker said.
The patient’s viral load showed a “nice response” over the next eight to 10 weeks, plateauing between 100 and 1,000 IU/mL, he said. At weeks 11 and 12 post-presentation, however, the patient’s viral loads rebounded to high levels while on ganciclovir. “We began to have significant concerns that resistance had developed in this individual.”
The clinical manifestations of CMV “can be nonspecific and difficult for health care teams to diagnose and manage, which makes diagnostic testing so critically important,” Dr. Binnicker said. CMV syndrome, which can include fever and elevated neutrophil counts, is a common manifestation. “When an individual presents with that category of CMV syndrome, testing is important.”
Other clinical manifestations are pneumonitis, hepatitis, and gastrointestinal disease with nausea, vomiting, or diarrhea, as was seen in the 67-year-old patient. “In some cases, central nervous system disease can also occur,” he said.

Dr. Binnicker
Prolonged exposure to an antiviral drug (median is five months), lack of immunity pre-transplant (donor-seropositive/recipient-seronegative), on a regimen of a strong immunosuppressive therapy, and inadequate antiviral drug delivery are risk factors for CMV antiviral resistance, Dr. Binnicker said. The incidence of antiviral resistance in CMV depends on the type of transplant and the patient, he said, but has been measured at between five and 12 percent in the solid organ transplant population and, depending on the study and population tested, between 1.7 and 14.5 percent among stem cell transplant recipients. Higher incidence rates are seen in recipients of lung (18 percent) and intestinal or multiorgan (31 percent) transplants.
Specific mutations in two CMV genes have been associated with antiviral resistance, Dr. Binnicker said.“The first and most common gene in which we see mutations associated with antiviral resistance is UL97,” which encodes for a kinase required for activation of ganciclovir and valganciclovir, one of the most common classes of drugs used to treat CMV in transplant patients, he said. Specific mutations occurring within UL97 “can prevent the phosphorylation or activation of those drugs.”
Mutations in the CMV gene UL54, which encodes for a DNA polymerase, are less common but can have a more severe outcome. These mutations are associated with resistance to the antiviral therapies cidofovir and foscarnet, in addition to ganciclovir and valganciclovir. “In some cases, there is cross-resistance to multiple drugs where we have fewer options for treatment in those patients,” Dr. Binnicker said. The mutation D301N, for example, can cause cross-resistance to ganciclovir and cidofovir.
“There are new classes of drugs coming out and we’re discovering there can be mutations in other CMV genes like UL56, or less commonly in UL89 and UL51, that can result in resistance to newer classes of drugs, such as letermovir,” Dr. Binnicker said. The list of mutations in UL56 is growing as more sequencing is done.
Persistent or recurrent high viral loads during prolonged (greater than six weeks) antiviral therapy raise suspicion for antiviral resistance. “As in the case highlighted, that individual had been on ganciclovir therapy for eight to 10 weeks and then showed a rise in their viral loads,” Dr. Binnicker said. A patient with viral loads that plateau at a high level, despite being on therapy, “can lead us down the road of thinking about a resistant population arising.”
Dr. Binnicker and colleagues at Mayo Clinic in Rochester developed a next-generation sequencing method for detecting resistance in CMV. The method, implemented for routine use in May 2019, requires a plasma sample from an individual who has had a viral load performed. “We’ve required that the viral load be 500 IU/mL or higher,” he said.
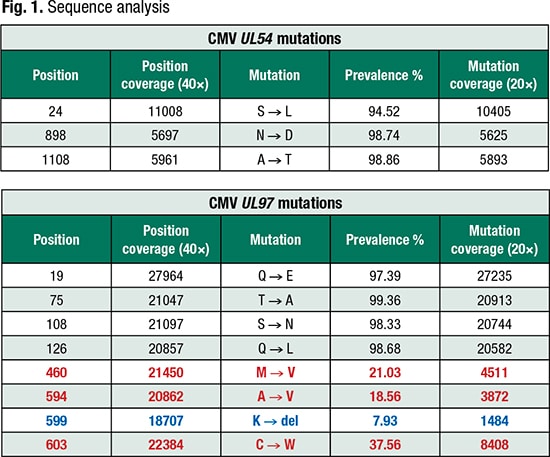
Mutations of interest based on CMV variant database (Bold red text: ≥15%); (Bold blue text: <15% but ≥ 5%)
“Once we have that sample, we send it through automated extraction to recover the DNA from the sample. Then we perform two separate PCRs on the viral nucleic acid” and amplify the entire UL97 and UL54 genes.
“We run a gel and check that the amplicon is the right size. Then we mix the products together, the UL54 and UL97 amplified material, into a standardized concentration and send it to our core next-generation sequencing facility, where the sequence is generated on the Illumina MiSeq.”
Once the sequence is completed, Dr. Binnicker’s team uploads the sequence data to Advanced Biological Laboratories, which has developed a bioinformatics analysis software program. “The software interrogates that sequence file and compares it with a reference database—wild-type CMV that lacks resistance-associated mutations.”
Within 30 minutes, he and his team receive a report from ABL summarizing whether there is predicted resistance or susceptibility to cidofovir, foscarnet, or ganciclovir (Fig. 1). “We can dive into the data more and look at the specific mutations that were identified in comparison with the reference database,” Dr. Binnicker said.
The CMV mutation analysis report lists all mutations identified in UL54 and UL97 and distinguishes the mutations associated with antiviral resistance, which are further distinguished by red text if present at a threshold of 15 percent or higher and by blue text if they are at low levels, between five and 15 percent of the reads.
“We wanted to call out those low-level mutations, those coming up between five and 15 percent, for monitoring and discovery purposes,” Dr. Binnicker said. “If we see in a patient that a resistance mutation is present at, say, 7.93 percent, we wanted to call that mutation out so that we can track it over time. We can see then how that viral mutation emerges or evolves as that patient is on antiviral therapy.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
