One population of special interest are those whose samples are normal for p-tau, t-tau, and p-tau/Aβ42 but abnormally low for Aβ42 only. The frequency is 19 percent for the Mayo Clinic laboratory and 16 percent for the reference laboratory. “Our thinking initially was that this is perhaps a result of preanalytic problems”—adhesion or absorption into the tubes, less volume, or inappropriate collection, he said. But a study of the data revealed “a different pattern that could point us either way.”
They therefore undertook a study of 535 patients who had Elecsys CSF biomarker testing over a one-year period. A neurologist who was blinded to imaging and CSF test results retrospectively assigned a clinical diagnosis. Associations between the blinded clinical diagnoses and CSF biomarkers were carried out via a Chi-squared test of independence.
Isolated abnormal Aβ42 is nonspecific, Dr. Bornhorst said. “There is the problem of preanalytical issues, but there is also something called normal pressure hydrocephalus.” It is a rare disorder, but in that patient cohort, 52 percent had an abnormal NPH. “In that disorder you get an increase of CSF volume,” he said, “and we think that drives down the overall Aβ42 concentrations” (Li W, et al. Presented at: American Academy of Neurology Annual Meeting; April 2–7, 2022; Seattle. S2.005). This points to a need for reporting quantitative markers rather than just the resulting ratio, he said.
Most of the patients with other clinical diagnoses, such as AD-related dementia, had an abnormal ratio, and a few also had an abnormal Aβ42 level. “So we did association studies,” he said, and “an abnormal ratio was strongly associated with AD dementia.”
“NPH had a very strong association with Aβ42 being the only discrepant or abnormal marker.”
Mayo launched in May 2022 the Fujirebio Lumipulse G β-Amyloid Ratio (1–42/1–40) in vitro diagnostic test as an FDA-approved assay for use in patients age 55 and older.
When the FDA-cleared Roche ratio became available in December 2022, which was the ratio Mayo had been using in the Gen 1 assay, a recalibration was needed because of the change from Gen 1 to Gen 2. The new Gen 2 assays were slightly different and resulted in a slightly different cutoff ratio for the Elecsys assays Mayo had been using in order to maintain the previously validated clinical performance of the assay.
The FDA indicated that for each company’s assay, the laboratory can report only the ratio, in patients age 55 and older, and as a positive or negative result, or likely positive in the case of the Fujirebio assay.
The Mayo Clinic laboratory team compared the two ratios—p-tau/Aβ42 (Gen 1) and Aβ42/40—in a study of 150 individuals and found them to show similar agreement with amyloid PET (Campbell MR, et al. Alzheimers Dement. 2021;13[1]:e12190). “We had 97 percent concordance between the [Lumipulse] Aβ42/Aβ40 ratio and the [Roche] p-tau/Aβ42 ratio,” Dr. Bornhorst said, and “when we expanded that, they both showed equivalent performance with amyloid PET.”
Moving to the FDA-cleared Roche p-tau/Aβ42 ratio raised several considerations, one of which was the change from Gen 1 to Gen 2. The others were the more restrictive predefined collection protocol, a restandardization of the Aβ42 assay with an approximately 19 percent decrease at the reference value, and an increased biotin interference threshold for Aβ42 and p-tau.
Mayo implemented the Roche Elecsys Gen 2 assays in June but retained the laboratory-developed test classification because of the age cutoff, “which we felt was restrictive,” he said, and because they felt their existing preanalytic protocols were working well. In addition, Mayo neurologists wanted the quantitative results, “which was prohibited by FDA in an FDA-approved assay.”
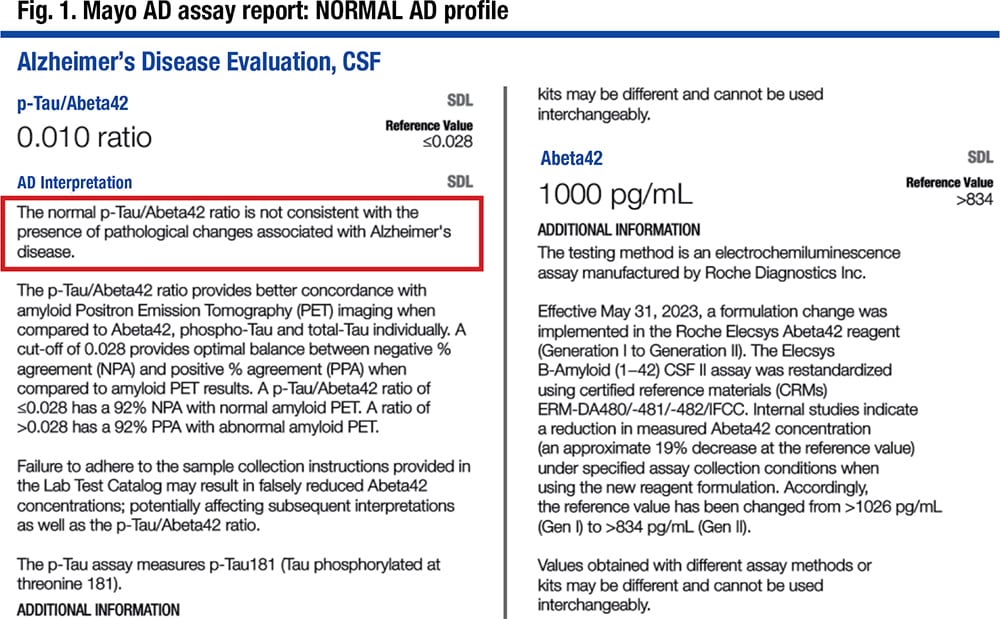
“In our population,” he said, “we adjusted the cutoffs based on the method comparison between Gen 1 and Gen 2 back to our original studies to maintain what we felt was equivalent diagnostic performance” (Fig. 1).
The three methods used to evaluate patients with cognitive decline for Alzheimer’s disease vary in performance.
Clinical diagnosis has a sensitivity of about 80 percent and a specificity of about 70 percent, Dr. Bornhorst said. Amyloid PET sensitivity is at 90 percent; specificity is at 84 percent. While CSF biomarkers require a collection technique many patients would rather avoid, the biomarkers have a sensitivity of about 92 to 96 percent and a specificity of about 88 to 90 percent.
“All three play a role in the establishment of Alzheimer’s disease,” Dr. Bornhorst said. No method is used in isolation, and clinical evaluation remains important regardless of the diagnostic methods used in conjunction.
CSF biomarker testing “is not meant for screening,” or for patients not considered to be at risk for AD, he said. Symptoms of REM sleep behavior disorder are not reason to test, he said, nor is a determination of disease severity in patients already diagnosed with AD. “That may be changing off-label. We’ll see how this develops as drugs come on the scene and treatment monitoring progresses.”
Other inappropriate uses: in those who are apolipoprotein E 4 carriers with no cognitive impairment, in lieu of genotyping for suspected autosomal dominant AD, and in autosomal dominant AD mutation carriers, with or without symptoms.
“Additional work needs to be done in the real world to further characterize the performance in retrospective and prospective clinical cohorts of patients with mild cognitive impairment,” Dr. Bornhorst said.
Amy Carpenter Aquino is CAP TODAY senior editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
