
Dr. Homer
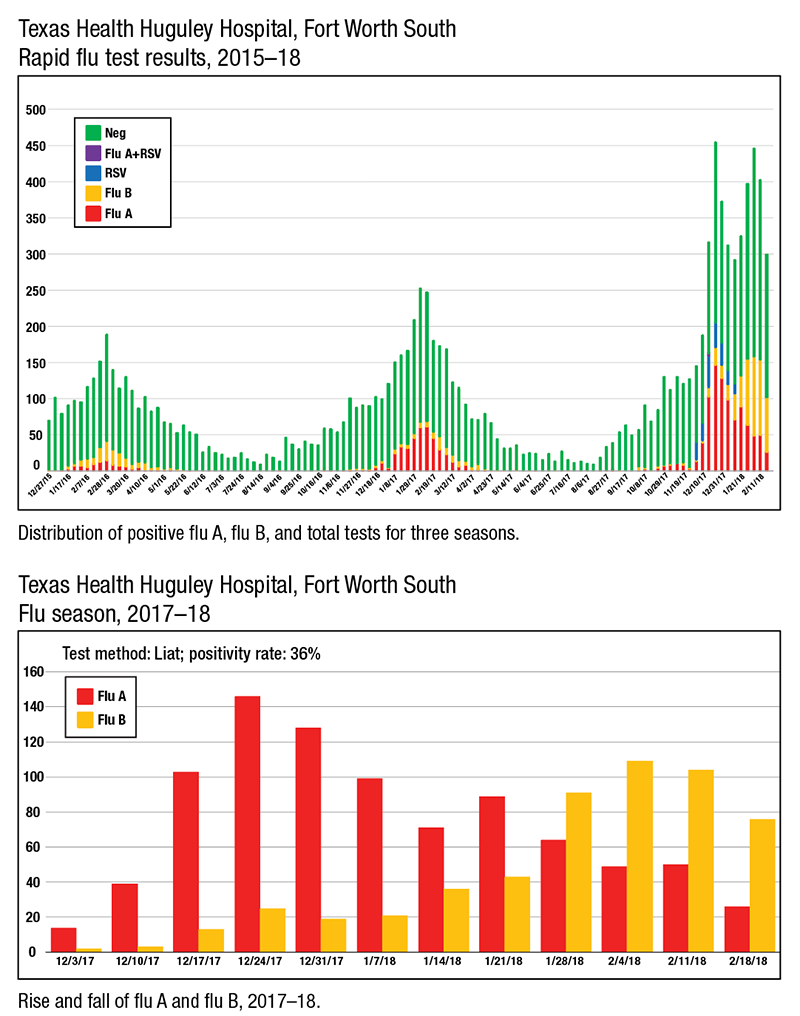
“It peaked the week between Christmas and New Year’s,” says Dr. Homer, who is chair of pathology and laboratory medical director at the hospital. “We ran more flu tests than we have ever run before in the history of our laboratory—over 450 flu tests that week.”
Of those 450 tests, 45 percent were positive for influenza or RSV. “Most of those positives were flu A,” Dr. Homer said in a Jan. 31 interview.
Dr. Homer, president of Huguley Pathology Consultants, has also noticed a slow but steady and significant rise in flu B since early December. “The first week of flu season, we had two [flu B] positive results, and last week we had 43.”
This flu season marks Huguley Hospital’s transition to rapid molecular PCR testing with the Cobas Liat system, a decision the laboratory and medical teams reached after two years of planning and conducting trials on several different platforms. “This is the only flu test we offer right now,” Dr. Homer says, adding that clinicians had grown frustrated with the unreliable results of the rapid antigen test used previously.
Satisfaction with flu test results is up substantially since the laboratory implemented the Liat on Nov. 30, 2017. “The ED doctors and the pulmonologists in primary care are saying, ‘We love this new test because the results match our clinical impression,’” Dr. Homer says. Microbiology specialists and laboratory generalists run the tests 24/7 and turn them around in about 30 minutes.
 Huguley Hospital is taking advantage of what Dr. Homer calls a “bonus” testing option of the Liat: the Cobas Influenza A/B and RSV assay.
Huguley Hospital is taking advantage of what Dr. Homer calls a “bonus” testing option of the Liat: the Cobas Influenza A/B and RSV assay.
“There was discussion about whether it was appropriate to test adults for RSV,” Dr. Homer says. “The pulmonologists especially thought that would be very helpful to them. It was their belief that RSV among adult patients with upper respiratory symptoms had a higher incidence than had been recognized.”
Testing adults for RSV by a rapid method was not an option until this flu season because the in-house RSV test kit used previously had been validated only for patients age 20 and under. Implementing the Cobas Influenza A/B and RSV assay on all patients who present with flu-like illness has validated pulmonologists’ assumptions. “The incidence of RSV among adult patients is higher than we had recognized previously,” Dr. Homer says. “It’s a minority, obviously, of our positive tests, but it’s a significant number of patients.”
Armed with more precise information, clinicians have cut down on the spread of RSV within the hospital by isolating admitted patients who test positive, which has also reduced absenteeism among nurses and other staff, Dr. Homer says. “We didn’t know what it was in years past. They were just getting sick.”
But momentum in the fight against RSV hit a Texas-sized roadblock during the third week of January when the laboratory ran out of flu A/B plus RSV assays, and Dr. Homer was told new supplies were not immediately available.
“Unfortunately, Roche has not been able to keep up with the demand for these kits, and as of last week, we had to switch to the flu A/B and not offer the RSV,” Dr. Homer says. “The clinicians are not happy about that, but there’s nothing we can do until the supply is replenished.”
In the meantime, the laboratory is using the RSV test kit it formerly used for patients age 20 and under and sending out RSV tests for older patients as needed.
Roche apologized for the flu A/B plus RSV assay shortage but said it would be several weeks before the laboratory could expect a new shipment. “Probably when the flu season is over, to be honest,” Dr. Homer says.
In response to a CAP TODAY inquiry, Roche on Feb. 6 said it was experiencing unprecedented demand for its flu A/B plus RSV test for the Cobas Liat platform in a season in which cases of influenza-like illness have reached historic levels, second only to the 2009 pandemic. “We’re excited about the market adoption of our new point-of-care PCR technology, but the demand has created a temporary supply interruption for our flu A/B plus RSV assay. We expect to have it back in stock shortly,” said Nate Patton, director of marketing for the Cobas Liat system.
“They did not anticipate the demand for this test,” Dr. Homer says. “I think they anticipated that sites using their tests would implement both the flu A/B and the flu A/B plus RSV options, and the clinicians would only choose to add the RSV option on younger patients.” And that’s on top of the unusually heavy flu season.
Despite the temporary setback, the advantages of rapid molecular PCR testing are clear, even partway through the flu season. Positivity rates for flu A, flu B, and RSV were at 37 percent as of Feb. 1, compared with a 17 percent positivity rate using the rapid antigen test during the 2016–17 flu season.
“We’re certainly detecting more patients with disease, and it has a higher correlation, at least anecdotally, with what the clinicians are seeing,” Dr. Homer says.
“The point of this project was to demonstrate we could serve the ED with a rapid molecular flu test that they could rely on,” he says. Inventory issues notwithstanding, “I think we’ve done that. If we can get the inventory problems corrected for next flu season, I think we’ve moved the bar on testing.”
More than 100 nursing homes in northwest Louisiana, east Texas, and southern Arkansas rely on the testing services of Doctors Lab, an independent clinical laboratory in Shreveport. “We have never had a year before like this, ever,” says owner Donna Poimboeuf, BSMT(ASCP).
Since detecting the first positive flu results in early December, Poimboeuf and her staff have been using Meridian Bioscience’s Tru Flu kit to test specimens 24 hours daily. When laboratory staff run short of the Tru Flu kits, they supplement with another rapid antigen test, the BioSign Flu A and B from Germaine Laboratories.
Her clients’ need for rapid turnaround times has been so great, Poimboeuf says, that nursing home directors cannot wait for the phlebotomists’ regular, predawn appointments to collect specimens. “They want to know immediately so they can start quarantining and treating them,” she says of the residents. “The first time we tested, we were getting 19 or 20 people positive. In a nursing home, that’s really bad.”
One challenge has been keeping laboratory staff healthy. “I’ve had quite a few employees who have tested positive for flu,” Poimboeuf says. It required her to add staff and hours to the laboratory’s schedule, in addition to adding staff to night and weekend shifts to handle the unusual load. “We’re almost running a full shift after hours.”
Poimboeuf recommends that all phlebotomists practice universal precautions. “A lot of our phlebotomists used to laugh at the whole mask thing,” she says. “Well, they don’t laugh anymore.”
Although northern California has not seen quite the same level of flu activity as other areas of the country, the number of flu tests across the University of California Davis Health system doubled from December to January.
“We’re seeing the increase in the West Coast,” says Nam K. Tran, PhD, associate professor of clinical pathology and director of clinical chemistry, toxicology, and point-of-care testing at the UC Davis School of Medicine. “The CDC said flu season is peaking, but California can lag behind the East Coast.”
Laboratory staff reviewed flu data as far back as 2009 for comparison. “This isn’t as bad as the H1N1 from 2009, but this is obviously one of the more severe years with a predominantly—based on our molecular test results—H3N2 virus,” he says. He notes that different variants of the H1 virus are the next most commonly seen virus, with a rare instance of the H1N1 from 2009 detected.

Dr. Tran
Dr. Tran spoke with CAP TODAY one week after the UC Davis Medical Center went live with molecular point-of-care testing for flu in the ED with the CLIA-waived Cobas Influenza A/B assay on the Roche Cobas Liat system. “We actually did the transition just as the season began to get worse,” says Dr. Tran, who credits planning and teamwork for the smooth switch from rapid influenza diagnostic testing and administrators who heeded the lab’s warning that better technology was needed.
“I will quote our ED nurses since they bear the brunt of any testing during flu season,” Dr. Tran says: “‘We aren’t stressed out, or feel we are running low on supplies, or overwhelmed.’”
He reports a surplus of flu tests and judicious use of anti-influenza drugs in the ED: “We’ve been navigating it quite well.”
In addition to the Liat, UC Davis relies on the respiratory viral panels of GenMark Diagnostics and BioFire. RSV testing on the BioFire instrument is limited to ED patients as part of a quality project. “As recommended by guidelines, treatment for RSV is supportive care. If you’re a high-risk patient with RSV, you’re probably going to the emergency room in the first place, so you don’t need this test in the clinics,” Dr. Tran says.
“But for flu testing, if you only have a question of ‘Does this patient have the flu or not?’ and it is not necessary to differentiate between other pathogens such as rhinovirus, then by all means run the Liat,” he says. “It’s far more cost-effective and time-effective to run a 15- to 20-minute test at the point of care in the ED or clinics versus sending it to the clinical lab for the full respiratory panel, which could take a couple of hours for same-day results.”
One common exception is pediatric patients who exhibit symptoms that overlap with whooping cough. “We would recommend, especially for high-risk children, to do the [BioFire] panel, or similar platforms, because we can actually test for Bordetella pertussis using these devices,” Dr. Tran says.
The core laboratory’s commitment to switching to molecular testing for flu dates back to the 2009 H1N1 swine flu pandemic. “UC Davis was one of the few places that recognized back in 2009 that immunoassay tests were inadequate for swine flu detection,” Dr. Tran says.
Accuracy for the previously available tests varied widely, ranging from 10 to 70 percent, depending on the population, says Larissa May, MD, MSPH, MSHS, professor of emergency medicine and director of ED antibiotic stewardship at UC Davis School of Medicine. “Typically, for the patients for whom we were most needing to know the diagnosis, the test performed relatively poorly.”
That includes high-risk patients, for whom the CDC recommends antiviral treatment, even with a negative rapid antigen test result. “That made the test not very useful, and a lot of folks were either not diagnosing or not testing,” Dr. May says. “Now that we have this accurate test, we can use it in our high-risk patients to determine who needs treatment or whether high-risk household contacts should be considered for prophylaxis.”
Dr. May calls molecular POC testing a game changer for influenza diagnosis. “The sensitivity is pretty close to 100 percent for influenza, and the specificity is very good as well.”
The 20-minute test run time on the Cobas Liat is “not unreasonable” for a diagnostic test, she notes, even for patients being discharged from the ED.
When it came to training nearly 200 UC Davis Health emergency nurses to perform molecular flu tests, clinical nurse educator Jennifer Mattice, MS, RN, says she focused on consistency in training and cleanliness in test performance. “You have to be careful that you don’t contaminate the specimen and get a false-positive,” she says, adding that it was one of her “biggest teaching points.” By early February, nearly 90 percent of nurses were trained on one of the two Liats in the ED.
“The staff were excited to learn something new, and they respected that this was a better type of flu testing than what we had before,” Mattice says.
Aligning the Liat devices with the UC Davis antibiotic stewardship program is underway. “That’s starting as we speak and in parallel,” Dr. Tran says. “Stay tuned.”
[hr]
Amy Carpenter Aquino is CAP TODAY senior editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
