Whole blood use and prehospital transfusion
April 2023—There have been several recent articles in CAP TODAY regarding the use of whole blood and prehospital transfusion.1-3 The general tenor of the reporting has supported these novel practices. We would like to suggest an alternative perspective.
There is limited evidence that whole blood is better than component transfusion. In fact, use of whole blood in the military demonstrated similar dilutional effects as red cell unit transfusion.4,5 A recent systematic review and meta-analysis of existing studies also did not support a benefit of whole blood over component therapy.6 The systematic review identified only five small, randomized trials, including a single randomized trial in trauma patients7 that found no difference in patient-important outcomes. This year, a large retrospective observational analysis was published of 2,785 trauma patients across Canada and the United States managed with and without whole blood.8 The authors found that patients administered a median of one unit (interquartile range 1-1; only five percent of patients received more than one unit) of whole blood, in addition to component therapy, had a lower 24-hour (hazard ratio, 0.63; 95 percent confidence interval, 0.41–0.96; P = .03) and 30-day mortality (HR, 0.53; 95 percent CI, 0.31–0.93; P = .020). The whole blood group baseline characteristics were markedly better than the component patients. Despite noting the limitations of their retrospective study design and the lack of biological plausibility of a single unit of whole blood making such a marked improvement in survival, the authors concluded that their analysis should lead to prioritizing the use of whole blood in traumatically injured patients.
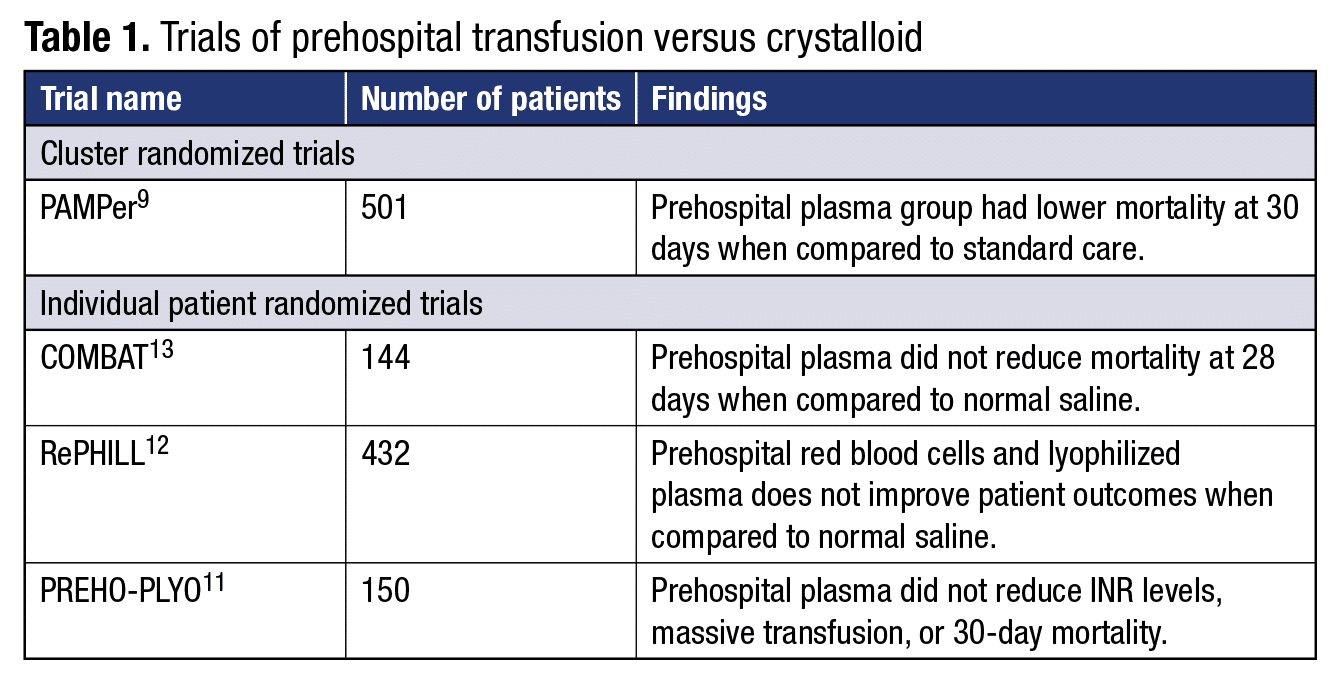
In regard to prehospital transfusion, while the December 2022 article1 cited the PAMPer trial9 as demonstrating benefit, interpretation of this study is hampered by several factors. These include the cluster randomized trial design, “futility bias,” and the low survival of patients in the control group when compared with previous studies.10 In fact, three other individual patient randomized trials without the above limitations have shown no benefit to prehospital transfusion (Table 1).11-13
There are also several potential harms from prehospital transfusion and the use of whole blood. As there is a constant shortage of O negative blood donors in the country, most prehospital programs will have to supply O positive whole blood or RBCs for transfusion. If a person of childbearing potential happens to be RhD negative and receives an RhD-positive unit of whole blood or RBCs, that person has an approximately 30 percent chance of forming an alloanti-D antibody and therefore is at risk for hemolytic disease of the fetus and newborn (HDFN) due to anti-D in a future pregnancy.14 The care of a pregnant patient with anti-D with an at-risk fetus is not trivial.10 More than 75 percent of obstetricians refer patients at risk for HDFN to the maternal-fetal medicine referral centers throughout the United States.15
 In addition, whole blood has a shorter outdate than components, must be derived from a male donor, and must test negative for high titers of anti-A and anti-B isohemagglutinins. Component therapy also allows for a single donation to benefit multiple patients. Furthermore, use of O whole blood as a universal product exposes patients to anti-A and anti-B isohemagglutinins. The potential impact of this incompatibility is still being explored.16
In addition, whole blood has a shorter outdate than components, must be derived from a male donor, and must test negative for high titers of anti-A and anti-B isohemagglutinins. Component therapy also allows for a single donation to benefit multiple patients. Furthermore, use of O whole blood as a universal product exposes patients to anti-A and anti-B isohemagglutinins. The potential impact of this incompatibility is still being explored.16
There may be certain circumstances in which prehospital transfusion and use of whole blood are warranted (e.g. remote/rural locations), and further research may help clarify. Indeed, the U.K. has launched the SWIFT trial that will enroll 848 patients and compare prehospital whole blood to component therapy.17 At this time, however, we believe there is limited evidence supporting the use of either whole blood or prehospital transfusion outside of randomized, controlled trials. In the setting of clear potential harm and a lack of benefit in randomized clinical trials, physicians need to carefully weigh the risks and benefits before implementing such programs as standard of care.
- Paxton A. The way forward for prehospital transfusion. CAP TODAY. 2022;36(12):1, 12, 14, 16, 18. https://bit.ly/CT_2022-36-12
- Rice S. One hospital’s story: ins and outs of low titer O whole blood use in trauma. CAP TODAY. 2022;36(7):26, 28. https://bit.ly/CT_2022-36-7
- Albert C. Lining up for low titer O whole blood in trauma care. CAP TODAY. 2022;36(6):5–6, 8, 11. https://bit.ly/LowtiterO
- Leslie SD, Toy PTCY. Laboratory hemostatic abnormalities in massively transfused patients given red blood cells and crystalloid. Am J Clin Pathol. 1991;96(6):770–773.
- Miller RD, Robbins TO, Tong MJ, Barton SL. Coagulation defects associated with massive blood transfusions. Ann Surg. 1971;174(5):794–801.
- Geneen LJ, Brunskill SJ, Doree C, Estcourt LJ, Green L. The difference in potential harms between whole blood and component blood transfusion in major bleeding: a rapid systematic review and meta-analysis of RCTs. Transfus Med Rev. 2022;36(1):7–15.
- Cotton BA, Podbielski J, Camp E, et al.; Early Whole Blood Investigators. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258(4):527–532.
- Torres CM, Kent A, Scantling D, Joseph B, Haut ER, Sakran JV. Association of whole blood with survival among patients presenting with severe hemorrhage in US and Canadian adult civilian trauma centers. JAMA Surg. Published online Jan. 18, 2023. doi:10.1001/jamasurg.2022.6978
- Sperry JL, Guyette FX, Brown JB, et al.; PAMPer Study Group. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–326.
- O’Brien KL, Shainker SA, Callum J, et al. Primum, non nocere: whole blood, prehospital transfusion and anti-D hemolytic disease of the fetus and newborn. Transfusion. 2023;63(1):249–256.
- Jost D, Lemoine S, Lemoine F, et al.; Prehospital Lyophilized Plasma (PREHO-PLYO) Study Group. Prehospital lyophilized plasma transfusion for trauma-induced coagulopathy in patients at risk for hemorrhagic shock: a randomized clinical trial. JAMA Netw Open. 2022;5(7):e2223619.
- Crombie N, Doughty HA, Bishop JRB, et al.; RePHILL collaborative group. Resuscitation with blood products in patients with trauma-related haemorrhagic shock receiving prehospital care (RePHILL): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Haematol. 2022;9(4):e250–e261.
- Moore HB, Moore EE, Chapman MP, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 2018;392(10144):283–291.
- Seheult JN, Callum J, Delaney M, et al.; Biomedical Excellence for Safer Transfusion collaborative. Rate of D-alloimmunization in trauma does not depend on the number of RhD-positive units transfused: the BEST collaborative study. Transfusion. 2022;62(suppl 1):S185–S192.
- Van Kamp IL, Klumper FJCM, Oepkes D, et al. Complications of intrauterine intravascular transfusion for fetal anemia due to maternal red-cell alloimmunization. Am J Obstet Gynecol. 2005;192(1):171–177.
- McCullagh J, Cardigan R, Brunskill SJ, et al. Assessing the risks of haemolysis as an adverse reaction following the transfusion of ABO incompatible plasma-containing components—a scoping review. Blood Rev. 2022;56:100989.
- Study of Whole Blood in Frontline Trauma (SWIFT). NHS Blood and Transplant. https://www.nhsbt.nhs.uk/clinical-trials-unit/current-trials-and-studies/swift/
Kerry O’Brien, MD
Medical Director, Blood Bank
Department of Pathology
Beth Israel Deaconess Medical Center
Assistant Professor
Harvard Medical School
Jeannie Callum, MD
Professor and Director
Transfusion Medicine
Department of Pathology and Molecular Medicine
Kingston Health Sciences Center
Queens University
Kingston, Ontario
Scott A. Shainker, DO, MS
Annie and Chase Koch Chair in Obstetrics and Gynecology
Director, New England Center for Placental Disorders
Beth Israel Deaconess Medical Center
Assistant Professor
Harvard Medical School
Lynne Uhl, MD
Director, Division of Laboratory and Transfusion Medicine
Department of Pathology
Beth Israel Deaconess Medical Center
Associate Professor
Harvard Medical School
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
