HPV primary screening
November 2014—We read with great interest the two recent articles by William Check, PhD, highlighting primary HPV testing proposals (June and September 2014). Additional related information not covered in the two CAP TODAY articles should be brought to readers’ attention.
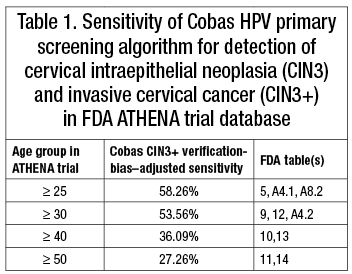
The first article focused on the ATHENA trial, in which a primary human papillomavirus screening algorithm based on the Roche Cobas HPV assay was evaluated. Although cervical cancer risk increases significantly with age (Cancer. 2014;120:2032–2038), no mention was made of ATHENA trial data documenting that the proposed Cobas primary HPV screening algorithm had not only limited overall verification-bias–adjusted sensitivity for detection of cervical intraepithelial neoplasia 3 (CIN3) and cervical cancer (CIN3+) but that this limited sensitivity steadily decreased even further among the highest risk older age groups (Table 1). The low documented rates for CIN3+ detection (27 to 58 percent) with the proposed Cobas algorithm intuitively raise questions about the safety of the algorithm and its use with extended screening intervals.
Source: 2014 meeting materials of the Microbiology Devices Panel. FDA Executive Summary: March 12, 2014. http://j.mp/microbiologydevicespanel.
The second CAP TODAY article focused on a recent publication analyzing risk for CIN3 and cervical cancer during a lengthy experience with conventional Pap and Hybrid Capture 2 (HC2) HPV cotesting in the Kaiser Permanente Northern California system (Gage JC, et al. J Natl Cancer Inst. 2014;106[8]:pii:dju153). While lead author Julia C. Gage, PhD, MPH, acknowledges in the CAP TODAY article that the statistically significant lowest risks for CIN2 and CIN3 were achieved with Pap and HC2 HPV cotesting, the difference in risk between cotest-negative and HPV-negative patients is dismissed as “small.” The CAP TODAY article goes on to add: “The difference in cancer risk was small and not statistically significant.” Although the JNCI authors have chosen thus far not to allow independent statistical evaluation of their privately held data set or to disclose most P values in the data comparisons, Dr. Gage clearly said in a public presentation in August at the 29th International Papillomavirus meeting in Seattle that the lowest cancer risk at three years after negative cotest results (0.007 percent) was “statistically significant” when compared with cancer risk after a negative HC2 HPV test (0.011 percent). The P value comparing the lower cancer risk at five years after negative cotest results (0.014 percent) with cancer risks after negative HC2 HPV test results (0.017 percent) is still undisclosed.
When the Pap test was introduced after World War II, the emphasis was on decreasing cervical cancer morbidity and mortality. Later, as new cervical screening technologies emerged in the late 1990s in response to observations on the limitations of screening, the Agency for Health Care Policy and Research noted that the verification-bias–adjusted sensitivity of the conventional Pap smear was only “near 50 percent, much less than generally believed” (AHCPR, Evaluation of Cervical Cytology, 1999), and that decreased cancer incidence and mortality would require some combination of increased recruitment to screening, increased screening test sensitivity, and more frequent screening. Available SEER data through 2011 documents that cervical cancer rates have continued to decline in the new screening technology era. As the new era of screening using HPV testing continues to develop and evolve, it is surprising to observe that a new proposed HPV primary screening algorithm with FDA-documented CIN3+ sensitivity “near 50 percent” is being portrayed as a significant advance and seen as the basis for a shift from a continued focus on further reduction of cervical cancer through screening to a new emphasis on 1) extending screening intervals, 2) achieving only “reasonable” cervical cancer risk (now apparently defined as risk associated with every three-year screening with the conventional Pap test), and 3) decreased testing and associated health care expenditures. As Dr. Gage said somewhat dismissively in Seattle, “You can always get a slightly lower risk of cervical cancer by reducing your interval or adding additional tests.”
R. Marshall Austin, MD, PhD
Chengquan Zhao, MD
Department of Pathology
Gynecologic Pathology Division
Magee-Womens Hospital of
University of Pittsburgh Medical Center
■ Mark Stoler, MD, cytopathologist and professor emeritus of pathology and clinical gynecology, University of Virginia, replies: I thank Dr. Austin and Dr. Zhao for their ongoing interest in the comparison between the potential of an HPV primary screening algorithm and cotesting. In my opinion, CAP TODAY and especially its reporter William Check, PhD, have done a superb job in presenting both sides of the argument in a balanced manner. In response to some of Dr. Austin and Dr. Zhao’s specific comments, I would briefly add the following:
Verification bias adjustments, where any detectable disease in a sample of patients with normal screening tests is then extrapolated to the whole population, always have a larger impact on apparent sensitivity than they do specificity. In the opinion of many epidemiologists, this impact is now understood to potentially be very misleading, but in general the relative rankings of the tests do not change.1,2 Thus in ATHENA, while the verification-bias–adjusted sensitivities “sound” low, the VBA sensitivity of cytology is lower than for HPV in all comparisons. Furthermore, as was pointed out in a prior online exchange,3 the figures quoted in the table in Dr. Austin’s letter are the VBA sensitivity of the algorithm, not the test. Unfortunately, individuals often confuse VBA and non-VBA statistics from different sources and compare apples to oranges, including Drs. Austin and Zhao. In numerous published studies, the unadjusted sensitivity of cytology is on the order of 50 to 60 percent, whereas the unadjusted sensitivity of a clinically validated HPV test is always on the order of 90 to 95 percent. As noted in reference No. 3, the published unadjusted sensitivity of the Cobas HPV test for ≥ CIN3 is 92.0 percent compared with 75.1 percent for liquid-based cytology. The performance of both HPV testing and cytology in ATHENA is similar to what was found in the only other large North American screening study, Mayrand, et al., who reported an unadjusted sensitivity of Hybrid Capture 2 of 82.8 percent and 57.7 percent for cytology for ≥ CIN 2.4
The decrease in sensitivity with age of all cervical cancer screening tests is not a new phenomenon, nor perhaps an unexpected one given the known regression of transition zone up the canal inducing sampling issues confounded by the known problem pathologists have with age-related mimics of HSIL, an issue we discussed in reference No. 5. Still, in head-to-head, apples-to-apples comparisons like ATHENA, HPV testing is superior to cytology in sensitivity with only minimal impact on specificity across all age groups. This fact, together with the data presented to and independently analyzed both by the FDA advisory panel and yet again by the FDA, strongly supports our belief that the time has come to consider seriously the benefits and simplicity of an HPV-based primary screening algorithm for cervical cancer screening. Such an approach is gaining worldwide acceptance and will become increasingly necessary as one considers the need to screen populations that have been vaccinated against HPV, where the performance of cytology will only degrade further.6 I am sure we can all agree that the United States should “only have the problem” of having to address how to screen a properly vaccinated and protected population.
- Wright TC Jr., Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206:46.e1–46.e11.
- Sasieni P. Estimating prevalence when the true disease status is incompletely ascertained. Stat Med. 2001;20:935–949.
- http://j.mp/stoler-thomas-athena
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007; 357:1579–1588.
- Stoler MH, Wright TC Jr., Sharma A, et al. The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. Am J Clin Pathol. 2012;137:295–303.
- http://j.mp/ncsp-renewal
■ Julia C. Gage, PhD, MPH, research fellow, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, and Thomas S. Lorey, MD, medical director of TPMG Regional Reference Laboratory, Kaiser Permanente Northern California Region, reply: We have some clarifications in response to the letter written by Drs. Austin and Zhao.
If a study is large enough, even small differences between very low risks can be statistically significant as seen in our findings from KPNC. When comparing the cancer risks after an HPV-negative result with cancer risks after a cotest-negative result at the same time point (i.e. three or five years), the risks have overlapping 95 percent confidence intervals. The three-year cancer risk after an HPV-negative versus cotest-negative result is statistically significant (0.011 versus 0.007, P=.03) while the five-year cancer risk after an HPV-negative versus cotest-negative result is not (0.017 versus 0.014, P=.11).
Importantly, statistical significance should not be conflated with identifying the optimal screening strategy. The decision of how often to screen with what tests is increasingly more complicated and there will always be opportunities to push further toward greater reassurance by using more screening tests or shortening intervals or both. At an extreme, women could be screened every six months with cotesting, but we all realize this would result in excessive identification of benign transient infections and associated changes. A balance must be struck between greater reassurance and the associated costs and harms. We turn to professional guidelines committees, which are best suited to such an endeavor.
Use HPV test in conjunction with Pap
Published data show that an HPV test can fail to detect nine percent to 20 percent of cervical cancers (Zhao C, et al. Evidence emerging for HPV-negative cervical cancer. CAP TODAY, January 2014). At BioReference Laboratories, a national clinical laboratory with specialized expertise in women’s health, cancer, and genetics, we examined relevant data over a one-year period. Our review disclosed that 66 of 733 (nine percent) Pap tests interpreted as suspicious for cancer (high-grade lesions, or HSIL) had a negative Roche Cobas HPV test. If these women had been tested only with the Roche Cobas HPV assay, their potentially precancerous condition (HSIL) would have been missed. In addition, a number of the women who had HSIL Pap results accompanied by a negative Roche Cobas HPV test had the more definitive test for cancer—a cervical biopsy. Of the abnormal biopsies, 10 percent showed squamous cell carcinoma of the cervix, 40 percent showed other precancerous high-grade lesions (CIN 2/CIN 3), and 50 percent had endometrial cancer. These cancers were detected based on a positive Pap test.
In these times of rising health care costs where findings based on population studies may change recommended medical practice guidelines for individual patients, any such changes must be fully vetted and appropriately balanced before they are implemented. For this reason we strongly believe that rather than replace the Pap test, the HPV test should be used in conjunction with the Pap test to assess a woman’s true risk of cervical cancer. While we welcome new advances in medicine, we should learn from previous data and gather additional information before changing our cervical cancer screening behaviors. In light of Pap testing having been enormously successful in reducing deaths from cervical cancer and the cervical cancer screening guidelines having been changed as recently as 2012, we believe more caution and research are warranted before additional changes are made to a screening algorithm.
James Weisberger, MD
Chief Medical Officer
James W. Sharp, MD
Clinical and Anatomical Pathologist
Vassar Brothers Medical Center
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
