Charna Albert
June 2022—For many blood suppliers, there is more enthusiasm for low titer O whole blood than there is an ability to make it, especially with the pandemic having made it harder than ever to collect. With greater use and a stocking of multiple sites versus one central site, “there are lots of folks lining up for the product,” said Julie L. Cruz, MD, senior medical director at Versiti Blood Center of Indiana.
Dr. Cruz spoke last fall in a CAP21 session about the product, the donors, the titer, and the supply. With her was Julie Katz Karp, MD, associate professor and director of transfusion medicine at Thomas Jefferson University Hospital, who presented on its use in trauma-induced coagulopathy and at Thomas Jefferson.

Dr. Karp
“Half of it is just thinking about it. The other half is figuring out how you’re going to use it and getting it,” Dr. Karp said of low titer O whole blood (LTOWB). (The Thomas Jefferson experience with LTOWB will be reported in the July issue.)
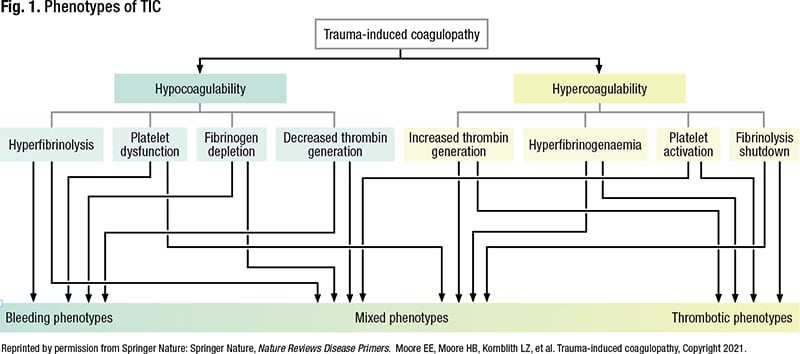
There is no standard definition for trauma-induced coagulopathy, a term established in 2010, but it refers to “abnormal coagulation capacity attributable to trauma,” Dr. Karp said. It manifests with a spectrum of phenotypes—anything from hypocoagulation to hypercoagulation (Fig. 1) (Moore EE, et al. Nat Rev Dis Primers. 2021;7[1]:30). What results is a function of many interactive factors, she said. “Things like tissue injury, the presence or absence of shock, and time from injury.”
In general, bleeding phenotypes and hypocoagulability are associated with early trauma-induced coagulopathy, and thrombotic phenotypes and hypercoagulability are more associated with later trauma-induced coagulopathy, “probably after initial resuscitation, to some degree.” But coagulopathy is a continuum, she noted. “Resuscitating people is not a straight line, and everyone is a little different.”
Trauma-induced coagulopathy can be treated with blood component therapy or LTOWB. Overall, a one-to-one-to-one ratio of blood components yields a dilute blood mixture compared with LTOWB, due primarily to the presence of anticoagulants and red cell additive solution in the individual components, and that’s problematic from a resuscitation perspective, Dr. Karp said. The one-to-one-to-one ratio of platelets, plasma, and red blood cells in blood component therapy “gets you something like reconstituted whole blood,” but compared with LTOWB, it has a lower hematocrit (29 percent versus 35 to 38 percent), a lower platelet count (90,000/µL versus 150,000–200,000/µL), and a lower whole blood concentration of coagulation factors (62 percent versus 85 percent).
Cold storage platelets are an emerging topic in trauma resuscitation, she said. “There’s a building body of evidence that refrigerated platelets are superior to room temperature storage platelets for acute hemostasis” (Cap AP, et al. Mil Med. 2018;183[suppl 2]:44–51). If kept cold, they may be more useful for patients who are actively bleeding, she said. “And that’s where we are with low titer O whole blood. These are patients who are actively bleeding,” so there’s a growing movement to keep platelets in cold storage. There’s evidence to suggest, too, that the hemostatic function of platelets in LTOWB is retained in refrigerated storage.
Should low titer O whole blood be leukoreduced? Though the majority of blood components in the U.S. are leukoreduced, Dr. Karp said, it isn’t required. The same dynamic applies to LTOWB—some is leukoreduced and some is not. Historically, whole blood has not been leukoreduced. But leukoreduction of blood components has become common in transfusion medicine and has been shown to have important benefits: lower rates of alloimmunization, fewer febrile non-hemolytic transfusion reactions, and less cytomegalovirus transmission.
“It’s a logical next step that we would leukoreduce low titer O whole blood when so much of our blood supply, blood-component wise, already is leukoreduced,” she said. When LTOWB is leukoreduced and isn’t transfused as whole blood, it can be further manufactured into leukoreduced red cell units (if leukoreduced before storage). “So leukoreducing low titer O whole blood sets you up to be able to make leukoreduced red cells, which is potentially a desirable thing from an inventory management perspective.” To leukoreduce LTOWB, there are data to support the use of platelet-sparing filters versus non-platelet sparing filters, and they’re now more commonly used in manufacturing LTOWB, she said.
A 2018 paper reported on an in vitro randomized controlled study that examined leukoreduced and non-leukoreduced whole blood and its respective hemostatic parameters (Remy KE, et al. J Trauma Acute Care Surg. 2018;84[6S suppl 1]:S104–S114). The authors measured median platelet concentration in 21 leukoreduced (with platelet-sparing filters) and 20 non-leukoreduced whole blood units stored at 4°C over 15 days. On day zero, immediately after leukoreduction, platelet concentration was lower in the leukoreduced whole blood. “What’s interesting is that at all other time points, out to day 15 plus, there were no differences in the median platelet concentration between the leukoreduced and non-leukoreduced whole blood,” Dr. Karp said. There’s a difference early in storage, but it eventually diminishes.
In another finding from the same study, on days zero and five, the leukoreduced whole blood units had decreased maximum clot firmness (measured with rotational thromboelastography) compared with the non-leukoreduced whole blood. “So similar to what we saw in the platelet count, but once again, there was no difference in percent reduction [of ROTEM-MCF] from day zero to day 15 between leukoreduced and non-leukoreduced whole blood.”
The authors also measured maximal amplitude by thromboelastography, finding no statistically significant difference between the non-leukoreduced and leukoreduced units on each day tested except for day zero, when maximal amplitude was reduced in the leukoreduced whole blood compared with the non-leukoreduced whole blood. Again, there was no difference in the percent reduction of maximal amplitude from day zero to day 15 between the leukoreduced and non-leukoreduced groups.
Leukoreduction performed with platelet-sparing filters, then, caused a reduction in some measures of hemostatic potential. “This reduction was most prominent in days zero to 10 of storage,” she said. “But those differences were gradually superseded by the effects of storage itself.” And at the end of the 15 days of storage, “all of these products look more or less the same.”
So it’s unclear if LTOWB should be leukoreduced, she said. There may be logistical reasons to do so, though it may not improve standard of care. “But in the end, it seems like it’s a wash. There’s no clear, better answer as far as hemostatic function,” though other factors may tip the scales.
In the same study, Remy, et al., looked at agitation of whole blood stored at 4°C and found that agitation is not needed to maintain hemostatic function.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
