Karen Titus
March 2013—For pathologists and clinicians alike, molecular testing can carry shades of a Pinter play: a seemingly straightforward situation with a whiff of discomfort, if not outright menace, and bedeviled by shifting characters and layers of meaning that defy snap interpretations.
Yet molecular testing is fast becoming part of standard repertoire in clinical laboratory testing, and pathologists who shy away from it aren’t doing themselves—or patients, or clinical colleagues, or the profession—any favors. Deciding what tests need to be ordered and interpreting results are sine qua nons of the pathologist’s job. That includes molecular testing, says Wendy L. Frankel, MD. “We don’t want clinicians talking about it at their meetings, then looking at us as technicians and ordering us to set up this or that,” says Dr. Frankel, distinguished professor of pathology, and vice chair and director of anatomic pathology, Ohio State University Wexner Medical Center (OSUWMC).
No indeed. Nor should the imminent arrival of next-generation sequencing be seen as a get-out-of-jail-free card. “If we start sequencing every colon cancer, a lot of what we do will change,” Dr. Frankel concedes. “But that’s OK. Everybody needs to know what to do in the meantime.” It’s not as if all labs will begin sequencing every tumor by month’s end.

For molecular colorectal cancer testing, next-gen sequencing is the future. At the University of Pittsburgh, where the future arrived last November, the lab runs an NGS panel of 46 genes and more than 740 mutations, says Dr. Marina Nikiforova, here with GI oncologist Nathan Bahary, MD, PhD. [Photo: Scott Goldsmith]
“It’s funny—it seems like there are probably 10, 15 different tests for any tumor,” he says. “But in fact, there are currently only a few with proven clinical utility for any given tumor type.”
With colorectal cancer, surgical pathologists don’t need a test to help establish a diagnosis. “That’s easily done with the microscope alone. So there’s really nothing to do there,” he says. For monitoring CRC recurrence, he knows of no molecular test.
The main areas in which molecular testing aids colorectal cancer patients now are identifying those who have the hereditary CRC syndromes known as Lynch syndrome familial polyposis and MYH-associated polyposis, identifying which patients with advanced CRC are candidates for anti-EGFR therapy, and predicting prognosis and guiding therapy, especially in those patients with stage II CRC.
Lynch syndrome is the most common hereditary CRC syndrome, and the first step in evaluating CRC patients for this disorder is to assess their tumor for defects in DNA mismatch repair (MMR), using microsatellite instability (MSI) testing and/or immunohistochemical analysis for expression of the four DNA MMR genes known as MLH1, MSH2, MSH6, and PMS2. If the MSI or DNA MMR immunohistochemical results show evidence of defective DNA MMR, then the patient may be evaluated further for Lynch syndrome by assessing a peripheral blood sample for germline mutations of one of the four DNA MMR genes responsible for Lynch syndrome.
Laboratories use various numbers of MSI markers, but most tend to use five markers, says Dr. Halling, who presents a course at USCAP titled, “Colorectal Cancer: Molecular Testing for the Surgical Pathologist.” If two or more of the five markers are positive, then the tumor is said to exhibit high-level microsatellite instability, or an MSI-H phenotype. If none of the markers show instability, the tumor is said to be microsatellite stable or to show an MSS phenotype. If only one of the five markers shows instability, then the tumor is said to show low levels of MSI, or an MSI-L phenotype. About 15 percent of sporadic CRC exhibit high levels of microsatellite instability, and these tumors have been shown—even after adjusting for stage—to have a better prognosis than CRC that are microsatellite stable. It’s also been recently discovered, says Dr. Halling, that stage II CRC patients whose tumors exhibit an MSI-H phenotype and who are treated with 5 fluorouracil (5FU), a standard adjuvant treatment, do no better than if they had been treated with surgery alone, suggesting that these patients should not receive 5FU.
IHC, too, is used to assess a CRC or other Lynch syndrome-associated tumor for defective DNA MMR. As noted, four genes are involved in encoding proteins that participate in DNA mismatch repair: MLH1 (3p21), MSH2 (2p22-p21), MSH6 (2p16), and PMS2 (7p22). Tumors in patients with Lynch syndrome have a germline mutation of one allele of a DNA MMR gene (for example, MLH1) and a somatic mutation in the other allele. The presence of two inactivating mutations results in defective DNA mismatch repair, Dr. Halling explains. This, in turn, increases accumulation of mutations across the genome, especially in microsatellites. Some of the mutations promote tumorigenesis by inactivating tumor suppressor genes or activating oncogenes. Most microsatellites are in noncoding regions of the genome and instability of these microsatellites does not contribute to tumorigenesis. “Nonetheless,” he says, “MSI is a convenient hallmark for identifying that a tumor exhibits defective DNA MMR.”
The goal, then, of assessing tumors for defective DNA MMR is first and foremost to identify patients with Lynch syndrome, but it can also be useful in predicting prognosis and guiding therapy in patients with sporadic CRC.
For colorectal cancer testing, is one—MSI or IHC—better than the other? Do you need both?“Surgical pathologists, of course, like immunostains, so I think the field is drifting toward doing immunostains,” predicts Dr. Halling, in part because they have the advantage of being accessible to almost any laboratory. Based on CAP proficiency testing subscriptions, he says, DNA MMR IHC has already surpassed MSI testing, even though it’s only been available for several years. “But microsatellite instability will continue to be an important complementary and for some cases more robust technique,” he says.

Dr. Frankel
The bottom line, according to Dr. Frankel, is that both are good ways to screen, though each may miss the occasional patient. “You have to make an institutional decision as to which one is the best way to go. They’re both very good.” Few places do both as a screen because it’s expensive. If a patient is clinically suspicious despite a negative test by one method, however, consider testing by the other method.
Regardless of method, pathologists need to convey some basic information to their clinical colleagues. “Clinicians might not understand that when we do microsatellite instability testing, we need both tumor and normal tissue,” says Dr. Halling. And not every clinician grasps that this testing is used to identify patients who are more likely to have Lynch syndrome but that it is not the last step. They oftentimes don’t understand that the next step, if results show either high microsatellite instability and/or loss of protein stain, would be to provide a peripheral blood sample for germline testing to look for mutations in one of the four DNA mismatch repair genes.
“They also need to be aware that the immuno-stains act as a surrogate genetic test,” Dr. Halling continues, something neither clinicians nor pathologists typically think about in relation to immuno-stains. Certain patterns of staining have a very high positive predictive value for the presence of a germline mutation. For example, a patient whose tumor shows loss of MSH2 and MSH6 has a very high likelihood of having a germline MSH2 mutation and thus a diagnosis of Lynch syndrome. Pathologists and clinicians should discuss whether informed consent is necessary. For her part, Dr. Frankel says such consent is unnecessary because these stains evaluate protein expression and not genes. All colon cancer patients at OSUWMC are educated about the tests prior to surgery.
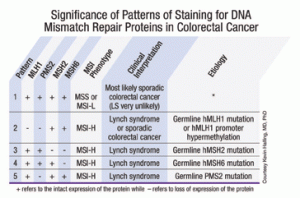 Pathologists should know how to interpret the clinical significance of the five common staining patterns (at right). When all the immunostains show normal expression, “it basically means that the tumor has normal DNA MMR and the patient is very unlikely to have Lynch syndrome.” In such a case, “you shouldn’t see microsatellite instability in the tumor,” Dr. Halling says. The most common abnormal pattern shows simultaneous loss of MLH1 and PMS2, with normal staining of MSH2 and MSH6. This could indicate either Lynch syndrome or a sporadic colon cancer. The other three abnormal staining patterns (MLH1/PMS2 normal, MSH2/MSH6 absent; MLH1/PMS2/MSH2 normal, MSH6 absent; and MLH1/MSH2/MSH6 normal, PMS2 absent) are highly suggestive of Lynch syndrome. The stains are also good at identifying which gene is likely to carry a germline mutation, and that can be used to guide subsequent gene sequencing.
Pathologists should know how to interpret the clinical significance of the five common staining patterns (at right). When all the immunostains show normal expression, “it basically means that the tumor has normal DNA MMR and the patient is very unlikely to have Lynch syndrome.” In such a case, “you shouldn’t see microsatellite instability in the tumor,” Dr. Halling says. The most common abnormal pattern shows simultaneous loss of MLH1 and PMS2, with normal staining of MSH2 and MSH6. This could indicate either Lynch syndrome or a sporadic colon cancer. The other three abnormal staining patterns (MLH1/PMS2 normal, MSH2/MSH6 absent; MLH1/PMS2/MSH2 normal, MSH6 absent; and MLH1/MSH2/MSH6 normal, PMS2 absent) are highly suggestive of Lynch syndrome. The stains are also good at identifying which gene is likely to carry a germline mutation, and that can be used to guide subsequent gene sequencing.
Dr. Frankel says her lab screens all resected colorectal cancers. In addition to helping identify patients (and possibly family members) with Lynch syndrome, it provides prognostic information—again, patients whose tumors are microsatellite unstable have a better prognosis than those with stage matched microsatellite stable tumors. It also predicts possible response to chemotherapy.
This approach grew out of a study she and her colleagues did at OSUWMC (Hampel H, et al. N Engl J Med. 2005;352:1851–1860) looking at more than 1,000 patients with colorectal cancer to determine whether it would be feasible and useful to screen all patients. The short answer: Yes. Subsequent studies have reinforced the point, Dr. Frankel says, as has the CDC’s Evaluation of Genomic Applications in Practice and Prevention (EGAPP) working group (Genetics in Medicine. 2009;11:35–41). The approach is being adopted by comprehensive cancer centers and academic centers, with community hospitals starting to follow.
The OSUWMC study found that one out of every 35 patients who have colorectal cancer has Lynch syndrome. For every patient, there will average an additional three family members who also have LS.
In the past, it was common to look for Lynch syndrome using Amsterdam and Bethesda criteria, looking at age, family history and, more recently, histology. “But you still miss patients,” she says.
If the IHC stains are intact, or if the microsatellites are stable—which will be true in about 85 percent of cases—it’s reasonable to halt further testing unless there’s strong clinical suspicion, says Dr. Frankel.
In the 15 percent of cases with MLH1 and PMS2 missing, three-quarters are due to a sporadic hypermethylation. “So, they’re not a genetic syndrome, but they’re still microsatellite unstable,” she says. The next step is to find out if LS is involved.
Some of the stains can be finicky, she cautions. In rectal cancer cases, MSH6 can be harder to read after neoadjuvant chemoradiation. Pathologists may see low levels of staining, or very weak staining, or even just the nucleoli. Many of these patients don’t have an MSH6 mutation. Dr. Frankel and her colleagues now circumvent the issue by testing the preoperative biopsy in resections with equivocal staining. In fact, post-treatment testing in rectal cancer cases is problematic in some cases because insufficient tumor may be available for testing. “More and more patients are getting neoadjuvant therapy for rectal cancer, and that can be challenging for some testing,” she says.
Molecular testing is nothing if not dynamic. Says Dr. Frankel: “Every month a new paper comes out, and we reassess what we do.” The MSH6 issue was just one example of the flexibility required of labs. “Initially we were going right to molecular sequencing after obtaining proper patient consent. We stopped doing that after, in five to 10 patients in a row, there was no MSH6 mutation.”
If MSI or IHC suggest that the patient might have Lynch syndrome, the pathologist will next want to consider testing for germline mutations, either in one or all of the DNA MMR genes—MLH1, MSH2, MSH6, or PMS2. Somatic BRAF mutations are generally not seen in tumors from those with Lynch syndrome, so BRAF mutation testing can be used to help filter out these cases from follow-up genetic counseling, testing, or both.Another option for determining if a patient with MLH1 and PMS2 loss has a germline MLH1 mutation, instead of BRAF testing, would be to look for methylation of the MLH1 promoter. If methylation is present, the tumor is presumed to be sporadic, and not due to Lynch syndrome. “So they don’t need additional testing,” says Dr. Frankel.
In patients with a BRAF mutation or methylation of the MLH1 promoter, LS can be ruled out in most cases. Once again, unless there’s strong clinical suspicion, “You can stop further testing,” says Dr. Frankel. If they don’t have a BRAF mutation or MLH1 promoter hypermethylation, then they’ll need additional testing. At this point, patients at OSUWMC are contacted by genetic counselors or nurses in the cancer genetics program, who obtain consent and discuss different testing options, which will vary depending on the patient’s history but basically involve some kind of gene sequencing.
Dr. Frankel’s lab started using BRAF mutation analysis routinely in 2009 in all MLH1-/PMS2-negative patients. It also occasionally offers MLH1 methylation testing.
KRAS testing is frequently performed on CRC patients as a way to determine which patients with advanced CRC are likely to benefit from anti-EGFR therapies such as cetuximab and panitumumab. BRAF testing is to a lesser extent performed for the same reason. Pathologists do need to decide which test to offer or recommend. And even once a choice has been made, it’s not necessarily chiseled in stone. Dr. Frankel says her lab has been getting requests for both KRAS and BRAF prior to treatment on patients who have metastatic cancer and are candidates for anti-EGFR therapy. “I’m letting the clinicians drive it, but some places are starting to feel getting BRAF and KRAS up front on the primary tumors may even change the initial drugs they use.”
Pathologists can choose from a variety of methods to assess for mutations in these genes. Controversies over the best method (see “KRAS mutations—which method is best?” CAP TODAY, May 2009) have yet to be resolved, says Dr. Halling, who also notes the FDA has shown a heightened interest in having labs use agency-approved assays for targeted therapies, rather than laboratory-developed tests.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
