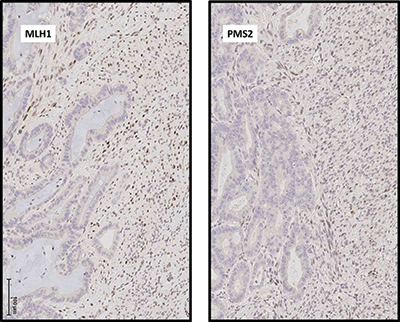
Fig. 2. (A) Immunohistochemical analysis demonstrates that expression of DNA mismatch repair proteins MLH1 and PMS2 is absent in malignant glandular epithelial cell nuclei but intact in malignant spindle cells. Photomicrographs taken at 200×.
Discussion. Leiomyosarcoma of the gastrointestinal tract is a rare tumor, and the finding of adenocarcinoma closely associated with a high-grade spindle cell proliferation raises two main differential diagnoses: carcinosarcoma, either primary or metastatic from a site such as the gynecologic tract, and collision of mucinous adenocarcinoma and leiomyosarcoma of the colon. The combined evaluation of IHC patterns and molecular findings in our case include distinct MSI status of the two separate tumor components and distinct mutational profiles. It is likely that these lesions originate from different clones and via different molecular pathways, and thus represent a collision tumor rather than carcinosarcoma.
This case represents the most extensive genetic analysis of a collision tumor of the colon yet reported, and it is the first report of NGS employed to analyze a collision tumor with carcinomatous and sarcomatous components. A previous report of a colorectal collision tumor included loss of heterozygosity in two of three microsatellite markers in a metastatic gastric carcinoma colliding with a primary rectal carcinoma with retention of all three markers. Both components of the collision tumor demonstrated microsatellite stability.1 A previous analysis of a thoracic collision tumor with pulmonary adenocarcinoma and malignant mesothelioma components included copy number analysis by single nucleotide polymorphism microarray and mutational analysis on a limited, amplicon-based NGS platform. Despite significant differences in genomic regional copy number, the NGS panel was too limited to identify a driver mutation in either tumor.2 The more extensive NGS panel used in our case allowed for identification of driver mutations in both tumors. Despite substantial evidence of mismatch repair deficiency in the adenocarcinoma, no loss-of-function mutation was identified in any mismatch repair gene. In such cases, mismatch repair deficiency may be caused by MLH1 gene promoter hypermethylation. Although the MLH1 promoter was not specifically interrogated in the current case, a tight association between activating BRAF mutation and MLH1 promoter hypermethylation is well established.3,4 As an activating mutation in BRAF was identified by NGS in the current case, MLH1 promoter hypermethylation is the likely cause of mismatch repair in the current case.
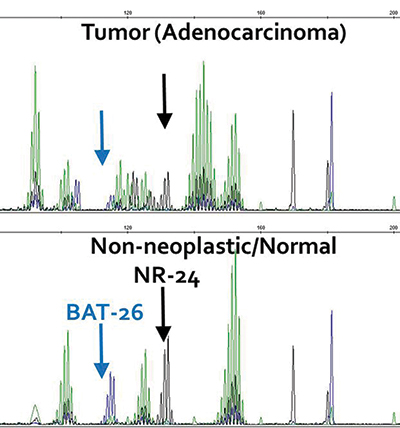
Fig. 2. (B) Microsatellite testing of the adenocarcinoma demonstrated instability of 5/5 mononucleotide repeats, two of which are highlighted here.
Therapy for collision tumors may need to be individualized for each tumor component. In this case, where neither component showed evidence of metastatic disease, surgical therapy alone was curative. Follow-up at one year after surgery did not show evidence of recurrence or metastatic disease. Thus, no further therapy was required.
In clinical practice NGS is used to provide predominantly predictive and prognostic information in most cancer cases. In this case, should the carcinoma metastasize, its MSI status would make the patient potentially eligible for immune checkpoint inhibition. Due to the presence of BRAF V600E mutation, the patient would be potentially eligible for trials of BRAF inhibitors and alternative chemotherapy combinations.5 Should the sarcoma recur or metastasize, the presence of an inactivating NF1 mutation could make the patient eligible for trials of MEK inhibitors and other therapies. However, in this case of a possible collision tumor, it is especially important to note that extended gene panel sequencing provided diagnostic information, in addition to predictive information, complementing morphologic and immunohistochemical analysis.
Methods. MSI testing was performed using the Promega (Madison, Wis.) MSI system according to the manufacturer’s instructions. Briefly, a polymerase chain reaction using fluorescently labeled primer amplifies seven microsatellite markers—five mononucleotide (NR-21, BAT-26, BAT-25, NR-24, and MONO-27) and two tetranucleotide (PENTA C and PENTA D) repeats.
NGS was performed using the Columbia Comprehensive Cancer Panel. The CCCP targets exonic and intronic sequences in 467 cancer-associated genes using DNA from formalin-fixed, paraffin-embedded tissue using Custom Agilent (Santa Clara, Calif.) SureSelect capture and Illumina (San Diego, Calif.) HiSeq 2500 sequencing. Sequences were aligned and nucleotide variants were called using NextGENe (SoftGenetics, State College, Pa.) software. Nucleotide variants were manually curated and classified after filtering with an in-house pipeline.
- Roh YH, Lee HW, Kim MC, Lee KW, Roh MS. Collision tumor of the rectum: a case report of metastatic gastric adenocarcinoma plus primary rectal adenocarcinoma. World J Gastroenterol. 2006;12(34):5569–5572.
- Naka T, Hatanaka Y, Marukawa K, et al. Comparative genetic analysis of a rare synchronous collision tumor composed of malignant pleural mesothelioma and primary pulmonary adenocarcinoma. Diagn Pathol. 2016;11:38.
- Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10(1):191–195.
- Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–793.
- Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19(2):187–225.
Test yourself
Here are three questions taken from the case report.
Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. Which of the following are included in the differential diagnosis of spindle cell lesions of the gastrointestinal tract?
a) Sarcomatoid carcinoma
b) Gastrointestinal stromal tumor
c) Metastatic leiomyosarcoma
d) Sarcomatoid mesothelioma
e) All of the above
2. Which of the following is not a gene that encodes for a mismatch repair protein?
a) MLH1
b) MSH2
c) MSH6;
d) BRCA2
e) PMS2
3. Activating mutation in the BRAF gene in colorectal carcinoma correlates most closely with which of the following?
a) Hypermethylation of the MLH1 gene promoter
b) Somatic loss-of-function mutation in the MLH1 gene
c) Germline loss-of-function mutation in the MLH1 gene
d) Loss of heterozygosity at the MLH1 gene locus
[hr]Dr. Heymann, Dr. Mansukhani, Dr. Sireci, and Dr. Sepulveda are in the Laboratory of Personalized Genomic Medicine, Department of Pathology and Cell Biology, Columbia University Medical Center (CUMC), New York, NY. Dr. Kiran is in the Department of Surgery, CUMC. Dr. Yang is in the Department of Pathology and Cell Biology, CUMC.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
