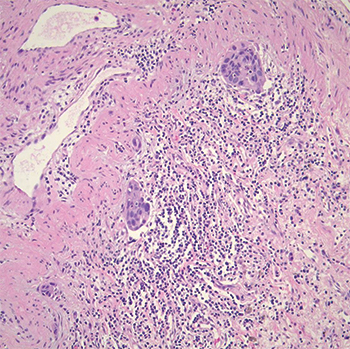
Fig. 2. Tumor demonstrating lymphatic vessel invasion.
Pathology. Histologic examination showed a moderately differentiated adenocarcinoma, acinar predominant with peripheral lepidic growth (Fig. 1). The tumor measured grossly 2.5 × 2.0 × 1.0 cm, and it extended to but did not invade the visceral pleural. The bronchial, vascular, and parenchymal margins were free of tumor. Lymphatic vessel invasion was identified (Fig. 2) but the 10 lymph nodes examined were negative. Staging was pT1b pN0.
Molecular. EGFR mutation analysis indicated that the tumor cells were positive for the L861Q point mutation in exon 21 and for the T790M point mutation in exon 20. KRAS mutational analysis was negative. Testing of a section of normal lung tissue showed positivity for the T790M mutation but was negative for L861Q. All testing was performed using FDA-approved methods.
Discussion. Despite the fact that KRAS mutations are much more commonly detected in pulmonary adenocarcinomas in smokers and EGFR mutations are more common in nonsmokers, the patient presented here had a wild-type KRAS but had a combination of two relatively rare mutations in the EGFR gene. The L861Q mutation makes up approximately two percent of all EGFR mutations whereas the T790M mutation is seen less than five percent of the time in the primary untreated tumor. Generally, when the T790M is detected in untreated patients, it almost always occurs in combination with a sensitizing mutation. The L861Q mutation is generally considered to be at least partially sensitizing to TKIs, though the agents currently available are not FDA approved for this mutation due to its rarity in the original clinical trial studies.
Classically, the T790M mutation arises only after treatment with first-line TKI of a tumor with an initial sensitizing mutation, accounting for 50 to 65 percent of acquired resistance. After development of the T790M mutation, the patients are then eligible for treatment with a third-generation TKI, with osimertinib currently the only FDA-approved therapy. Thus, the patient presented here had at initial diagnosis both a partially sensitizing and a resistance-conferring mutation in the EGFR gene and would not be expected to respond to first-generation TKI therapy. However, there is recent evidence that patients with initial pretreatment T790M mutations may respond to third-generation TKIs as first-line therapy, though they are not currently FDA approved for this purpose.
Although the patient has remaining unresected lesions in his lungs, he does not currently have known metastatic disease. Thus, he will be treated with active surveillance at this time. If the cancer does later present with metastasis, he will be offered treatment with a third-generation TKI as first-line therapy. Interestingly, in about half of patients with baseline T790M mutations in their lung cancer, the T790M may be present as an underlying germline mutation and result in a rare lung cancer hereditary syndrome, with an estimated 30 percent lifetime risk for developing lung cancer. Testing of normal lung tissue, which contained the T790M mutation but not the L861Q, provides evidence that the T790M mutation is likely germline in this patient. Genetic counseling and confirmatory peripheral blood-based germline EGFR mutation testing will be offered to the patient, especially given his positive family history of lung cancer.
- Sheikine Y, Rangachari D, McDonald DC, et al. EGFR testing in advanced non-small-cell lung cancer, a mini-review. Clin Lung Cancer. 2016;17(6):483–492.
- Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94(2):129–137.
- Gazdar A, Robinson L, Oliver D, et al. Hereditary lung cancer syndrome targets never smokers with germline EGFR gene T790M mutations. J Thorac Oncol. 2014;9(4):456–463.
- Oxnard GR, Miller VA, Robson ME, et al. Screening for germline EGFR T790M mutations through lung cancer genotyping. J Thorac Oncol. 2012;7(6):1049–1052.
Test yourself
Here are three questions taken from the case report.
Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. Precision therapeutic agents are currently FDA approved for lung adenocarcinomas possessing certain mutations/rearrangements in which of the following genes?
a) KRAS
b) EGFR
c) ALK
d) a and b
e) b and c
2. Approximately 90 percent of EGFR mutations include:
a) L861Q and G719X
b) T790M and L858R
c) Exon 19 deletions
d) L858R and exon 19 deletions
e) S7681
3. T790M mutations of the EGFR gene:
a) are seen only after initial treatment.
b) can be treated with osimertinib.
c) have only prognostic significance.
d) may exist as a germline mutation associated with hereditary lung cancer.
e) b and d
[hr]Dr. Khatib is a resident physician, Cheryl Bissaillon is supervisor of the molecular biology laboratory, Kim Lebel is manager of the molecular biology laboratory, Dr. Crisi is an associate professor, and Dr. Moore is an assistant professor and medical director, molecular biology laboratory—all in the Department of Pathology, University of Massachusetts Medical School-Baystate, Springfield.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
