
Dr. Bostwick
Dr. Bostwick has never seen a prostate in which he couldn’t locate “at least a small scattering of chronic inflammatory cells pushing on epithelium. More often than not,” he said, “you see a lot of inflammation.”
He and Dr. Iczkowski, an associate professor of pathology at Medical College of Wisconsin, published an autopsy study in 2003 showing that the amount of prostate inflammation was the same whether a male was age five or age 77, if the size of the organ was controlled for. Twenty-eight prostates were in the series. “It was a complete surprise” that they didn’t find any age-related differences, he said (Bostwick DG, et al. Prostate. 2003;55[3]:187–193).
Testosterone, of course, plays a critical role. According to the autopsy studies by W. A. Sakr and colleagues, 80 percent of men develop prostate cancer by age 80, he noted. That is, unless they were castrated before age 40. One study by Dr. Fang Liu Gu of Beijing found that none of the men in the royal palaces of China who were castrated before puberty had benign prostatic hypertrophy or prostate cancer.
“The idea is that by eliminating testicular testosterone, it eliminates the risk,” Dr. Bostwick said. “So testicular testosterone is a necessary but not a sufficient cause or factor required for prostate cancer.” The prostate is “a hormonally responsive organ with testosterone responsiveness. We know from animal studies that testosterone will drive oxidative stress.”
“Oxidative stress, we think, is a large part of this,” he said. “Genetic instability occurs, and then high-grade PIN and cancer. That’s the presumptive working hypothesis.”
Prostatic intraepithelial neoplasia, or PIN, is a cytologic abnormality with proliferative changes within preexisting ducts, ductules, and acini of the prostate, usually small- to intermediate-sized structures, he said. But there’s no invasion—no stromal involvement or new architecture outside of the acini. (Dr. Bostwick said he uses PIN and high-grade PIN interchangeably.)
In consensus conferences held largely in the 1990s, there was agreement to use the term “intraepithelial neoplasia” rather than “carcinoma in situ,” he said, because it wasn’t known if the changes to the prostate epithelium “arose right there locally or whether they spread through the plumbing.” Since then, he added, animal studies have suggested it does start at or near that site.
Dr. Bostwick displayed an image that he noted had high-grade PIN on the left and, on the right, normal prostate tissue from the same patient and viewed at the same magnification (Fig. 1). “And the difference is obvious. The reason you can tell it’s PIN even at low magnification is that the crowding of the nuclei creates this hyperchromasia at low power and the cytoplasm is also somewhat more dense. And, therefore, it can be observed even at low to intermediate magnification.” Numerous proliferative changes mimic PIN, however, so pathologists have to use higher magnification to confirm that, he cautions.
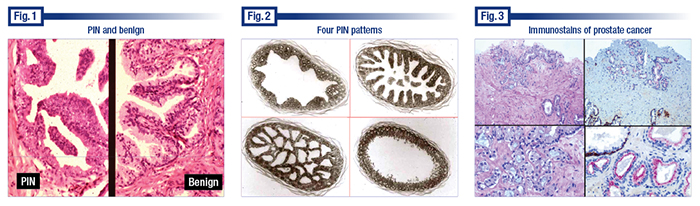 “Every cell has a nucleolus of some kind. Most often in the prostate, they are very small and punctate,” he said. “With PIN and cancer, the nucleoli tend to be large, sometimes very large,” and they tend to be pushing up against the nuclear membrane.
“Every cell has a nucleolus of some kind. Most often in the prostate, they are very small and punctate,” he said. “With PIN and cancer, the nucleoli tend to be large, sometimes very large,” and they tend to be pushing up against the nuclear membrane.
Seeing more than one nucleolus in a cell “is quite uncommon in the prostate,” Dr. Bostwick said. “It can occur normally but they are very, very tiny in that case. When you have multiple ones and they are large, that is almost certainly prostate cancer or PIN.”
There are four main patterns of PIN: tufting, micropapillary, cribriform, and flat (Fig. 2). “The micropapillary and cribriform may cross over,” he said. Most controversial is cribriform because it overlaps also with intraductal carcinoma. (For more on intraductal carcinoma, see page 44.)
“PIN does not have stromal invasion,” Dr. Bostwick said. “When there’s stromal invasion or early invasion, we call it cancer. Or if we aren’t sure we call it ASAP.”
“The reality,” he told pathologists, “is that you and I are going to confront cases in which we are absolutely, positively uncertain. These cases are always less than two dozen acinar structures.” Dr. Bostwick noted that Dr. Iczkowski did a study (in which Dr. Bostwick took part) showing that if there are more than two dozen, you can almost always call it benign or cancer (Iczkowski KA, et al. Arch Pathol Lab Med. 2000;124[1]:98–107).
Pathologists should be seeing an incidence of ASAP in the range of 1.5 to 2.5 percent, he said, “particularly in a world with immunohistochemical findings available” (Iczkowski KA. Ann Diagn Pathol. 2014;18[5]:301–311). According to virtually every paper published on the subject, the predictive value of ASAP for cancer is in the 40 percent to 60 percent range, he added.
“Sometimes,” Dr. Iczkowski told CAP TODAY, “minute foci of atypical glands are overcalled as definitive cancer when the hematoxylin-eosin stain and immunostain evidence suggest that an [ASAP] diagnosis would be more prudent. These same minute foci may also be underdiagnosed as benign.”
Dr. Bostwick’s laboratory uses four immunostains for prostate cases, among them cytokeratin 34B-E12 for the basal cell cytoplasm. “Some people use cytokeratin 5/6,” he said. “P63 stains nuclei in the basal cells.” Adenocarcinoma doesn’t have basal cells. “The cancer invades the stroma and basically the cells are growing wherever they want to grow. We think the basal cells are pushed aside because the cancer will grow between them.” Approximately one in 10,000 cases would be a basal-cell–based cancer, he said, “and that would look very different under the microscope.”
To help detect PIN and cancer cells, Dr. Bostwick uses racemase (P504S) for the cytoplasm. The fourth stain is the oncoprotein c-MYC, which stains the nuclei. “People haven’t adopted it widely yet,” he said of c-MYC. “There’s only one really good paper on it. So the papers haven’t been written.”
“c-MYC is seen in 100 percent of PIN and 97 percent of cancer nuclei. Not every nucleus. It’s usually seen in 50 to 80 percent of the nuclei, and it can also be seen in benign acini, but it’s invariably a very small number.”
Dr. Bostwick presented a case that convinced him immunostains can be used to cross over from ASAP to a cancer diagnosis in some instances (if consistent with light microscopy).
The case (Fig. 3) involved a small focus of a biopsy that Dr. Bostwick viewed as highly suspicious for cancer. Describing the features, he noted “a little bit of sort of pale wispy blue mucin which, while it is not specific for cancer, is certainly sensitive for some cancers and it sways me at least somewhat. It pushes me a little bit in that direction.”
Dr. Bostwick said he probably would have called the case ASAP if a triple immunostain hadn’t been so compelling. “The findings were absolutely consistent with cancer, which is to say the cytokeratin was completely negative. There were no basal cells around the focus. P63 was negative, so we have a second check and balance that there are no basal cells around any of the acini of concern.”
 The racemase (which he described as producing a distinctive Texas-red abluminal dot-like stain in this case) was positive in all the acini of concern. “We also had internal positive and negative controls [for cytokeratin and p63], and an internal negative control for racemase that was benign and nearby.” (c-MYC wasn’t yet available.)
The racemase (which he described as producing a distinctive Texas-red abluminal dot-like stain in this case) was positive in all the acini of concern. “We also had internal positive and negative controls [for cytokeratin and p63], and an internal negative control for racemase that was benign and nearby.” (c-MYC wasn’t yet available.)
Racemase can be positive in other findings, but if it’s strongly positive and limited to the acini that are thought by histology to be cancer, and p63 and the keratin are negative, that’s now sufficient by itself to diagnose prostate cancer, he said, assuming the histology doesn’t rule against it.
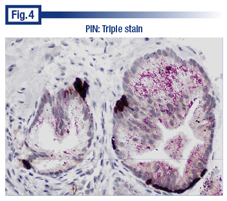
Dr. Bostwick presented two additional examples of triple stains, one of which was high-grade PIN (Fig. 4). “PIN is thought to be linked to cancer by virtue of the fragmented or discontinuous basal cell layer. You can see the beautiful basal cells, but there are only a handful of them and they are actually clustered in different areas. The racemase stain is quite distinctive in the luminal side of the epithelium where it usually resides,” he said. “And it raises the question of whether PIN is preparing to invade the stroma or not.” And while that’s believed to be the case and there is evidence from animal studies that it does happen, it can’t be proven using current methods in humans, he said. “So it’s still a strong, accepted hypothesis that PIN is a preinvasive lesion with cancer in the prostate.”
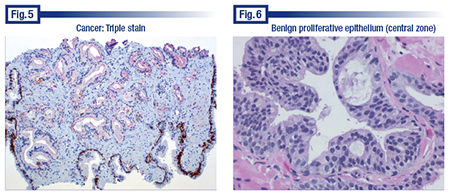
The other example of a triple stain was a case that Dr. Bostwick said pathologists could probably diagnose by light microscopy, except the nuclei were not particularly enlarged and hyperchromatic, nor were the nucleoli prominent. “So without the cytologic features of malignancy, notwithstanding the fact that we have maybe 12 to 18 abnormal acini in the middle of this, the supportive immunohistochemical findings with the nice positive controls at the periphery, I think, are sufficient together with the architecture to make the diagnosis of cancer—a flat-footed diagnosis, not suspicious but diagnostic.” (Fig. 5).
Dr. Bostwick also explained the pitfalls, starting with immunostains. He cautions that high-molecular-weight cytokeratin of any kind is often negative in atrophy or in atrophy with a lot of inflammation.
 Aberrant p63 staining can throw the unaware for a loop. In Dr. Bostwick’s experience, about one in 1,000 to 2,000 cancers is positive for p63. “The interesting thing is in these cases when it’s positive, in my experience, virtually every single nucleus is positive. It’s almost like a good positive control for p63,” he said. “They are terrifying cases because you expect them to be negative and support you and then you get this incredibly strong positivity.” P63 can also be patchy, and usually is in the benign prostate. “Not every single nucleus stains with p63, which is unfortunate.”
Aberrant p63 staining can throw the unaware for a loop. In Dr. Bostwick’s experience, about one in 1,000 to 2,000 cancers is positive for p63. “The interesting thing is in these cases when it’s positive, in my experience, virtually every single nucleus is positive. It’s almost like a good positive control for p63,” he said. “They are terrifying cases because you expect them to be negative and support you and then you get this incredibly strong positivity.” P63 can also be patchy, and usually is in the benign prostate. “Not every single nucleus stains with p63, which is unfortunate.”
Racemase, which is positive in cancer, can also be positive in benign epithelium. “It’s usually not nearly as strongly positive except in rare instances, and one of those is nephrogenic metaplasia, which is a known [benign] mimic,” he said.
Racemase can be negative in about a quarter of cases of atrophic cancer, hyperplastic prostate cancer, and the foamy gland variant of cancer, and those are the hardest ones of all to diagnose, he said. “c-MYC expression is seen in all of those actually very nicely, and it does increase with the Gleason score.”
The fixative can also be an issue. Looking at the nucleus to cytoplasm ratio is important because the nuclear size varies according to the fixative used, Dr. Bostwick said. “So if I use a different fixative, then [the specimen] can have huge nuclei and they are still normal. The amount of cytoplasm actually normalizes for the size of the nucleus. It controls for the effect of fixation, which is widely variable, depending on how you do it.”
“Just because the nucleolus is obvious or apparent to you under the microscope doesn’t mean it’s PIN or cancer,” he said, “because if the nucleus is also quite enlarged for whatever reason, it’s conceivable you are dealing with a mimic or a reactive change. We know radiation causes bizarre changes in nuclei and nucleoli. So in that setting be careful of the changes.”
In cancer or PIN, when the nuclei are enlarged, he said, so are the nucleoli, and most often disproportionately so. “Thus, the nucleus to nucleolus ratio is altered.”
Crush artifact, which results from pushing on the biopsy tissue to the point it alters the cells’ appearance, he said, may or may not cause the nuclei to appear larger. “It’s variable. Crush artifact causes the nuclear chromatin to become darker similar to prostate cancer.”
The central zone epithelium (Fig. 6) has arches or bridges and can thus mimic high-grade PIN. The central zone is the smallest of the prostate’s three zones. “So it’s not often sampled in prostate biopsies, and sometimes people over interpret it as PIN when it’s not. We don’t know why it’s a little more proliferative.” He cautions that it’s not a cribriform pattern of PIN or a cribriform pattern of intraductal carcinoma.
The most important prostate cancer mimic is post-atrophic hyperplasia, a variation of basal cell hyperplasia. Dr. Bostwick presented what he calls “the million dollar slide” from a case in which a pathologist misdiagnosed the benign condition as prostate adenocarcinoma many years ago (Fig. 7). When no cancer was found in the prostate after prostatectomy, the slide was reviewed and the error was discovered. The patient sued and received a settlement of about $2.5 million.
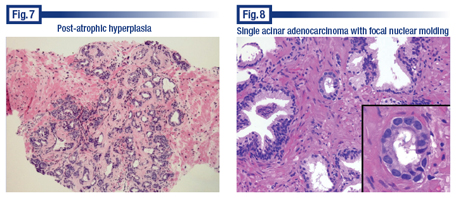 Dr. Bostwick wasn’t involved in the case and said he was not presenting it to impugn anyone but to demonstrate a mistake. He said the specimen has many of the features of prostate cancer. “It’s a proliferation of small- to intermediate-sized acini; it has glandular distortion. There’s variation of the size, shape, and spacing of the acini. It’s even set in a fibrotic stroma. But it turns out fibrotic stroma is quite uncommon in prostate cancer, unlike breast cancer and other cancers. It does occur, but it is not a diagnostic finding in prostate cancer for reasons we don’t understand.”
Dr. Bostwick wasn’t involved in the case and said he was not presenting it to impugn anyone but to demonstrate a mistake. He said the specimen has many of the features of prostate cancer. “It’s a proliferation of small- to intermediate-sized acini; it has glandular distortion. There’s variation of the size, shape, and spacing of the acini. It’s even set in a fibrotic stroma. But it turns out fibrotic stroma is quite uncommon in prostate cancer, unlike breast cancer and other cancers. It does occur, but it is not a diagnostic finding in prostate cancer for reasons we don’t understand.”
“The hyperplasia here is not an epithelial hyperplasia in the sense of proliferation within lumens,” Dr. Bostwick added. “It’s a hyperplasia of small acinar structures that are more than you’d expect in an area of this size.” He noted the atrophy where some of the acini had flattened, attenuated epithelium.
If they had done a stain for basal cells, he said, the patient wouldn’t have had an unnecessary surgical procedure. “The key to it is there are different types of post-atrophic hyperplasia and some will have a stroma that’s fibrotic and some will not. A fibrotic stroma is something that we pay a lot of attention to in other organs, particularly in breast cancer, but in the prostate, the fibrotic stroma is, first of all, much less likely and, secondly, it has less significance.”
Dr. Bostwick also presented a slide with a single acinar structure that he believed was cancer (Fig. 8). The case provided an example of nuclear molding, which he says is an uncommon finding in the prostate but usually a worrisome one when present. Nuclear molding “is when one nucleus is plastered against another and actually molds around it,” he said, adding, “It’s usually not a good thing to find for the patient.”
Dr. Bostwick said he saved for last what he calls “the worst and the best,” an atrophic adenocarcinoma that he almost missed (Fig. 9). “I’m going through the case and there are apparently only two worrisome acini,” he said, pointing them out at the top left of the image. But that’s not all. “It turns out that most of what you see in this image is actually malignant.” Dr. Bostwick noted what he described as a probable atrophic acinus at the top right of the image that was actually malignant. “The lumen is dilated by secretions pushing on it. The epithelium is flattened and attenuated, typical features of atrophy. And yet it’s malignant. There are no basal cells around almost all of the acini in this particular field.
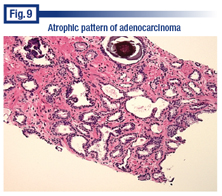 “The atrophic pattern of cancer may be racemase-negative in 25 percent of cases,” he said. “Fortunately, 75 percent are positive for that. c-MYC is fabulous for this. But the basal cells helped us more because there are no basal cells.”
“The atrophic pattern of cancer may be racemase-negative in 25 percent of cases,” he said. “Fortunately, 75 percent are positive for that. c-MYC is fabulous for this. But the basal cells helped us more because there are no basal cells.”
Whenever Dr. Bostwick is preparing to sign out a case that he thinks is going to be benign, he asks himself: “What am I about to miss? And at the top of my list is, Could it be foamy gland or atrophic cancer? If the answer is no, and I’m sure it’s not, then it’s good to go. I can sign it out as benign.”
Dr. Bostwick anchors himself with light microscopy. “And the immunostains are a very critical adjunct in select cases, but in the end it has to be the cumulative evidence. It’s also nice to look at it the next day, and you may find that you have a different eye in the morning than you do in the afternoon.”
He recommends a book by Daniel Kahneman, PhD (recipient of the Nobel Memorial Prize in Economic Sciences in 2002), Thinking, Fast and Slow (2011), in which the author talks about two systems of thinking. “System one is where you immediately react to something you see, and system two is where you thoughtfully think through the statistics, the background, the context.” System two is the mind’s slower, analytical mode.
“It’s really interesting,” he told pathologists, “because I think it speaks directly to what you and I do every day of our lives in looking at biopsies. And you may actually think differently at different times of the day.”
Dr. Bostwick is considering writing an article on or doing a study that applies Dr. Kahneman’s ideas to what pathologists do. “We are the ones who diagnose cancer,” he said. “We are the ones who ultimately, with our words and our eyes, label people definitively with a diagnosis that is more important than perhaps anything else in their lives.”
[hr]
Karen Lusky is a writer in Brentwood, Tenn. Dr. Bostwick will be a presenter at CAP ’16 on Sept. 28 in Las Vegas.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
