Q. Are there generally accepted guidelines for routine molecular testing of high-grade gliomas/glioblastomas (GBM)?
A. In short, the answer is no. However, molecular testing in gliomas is on the rise in routine clinical practice, which requires the practicing pathologist to be familiar with these genetic markers, the implications of such testing, and the techniques employed for their detection.
Molecular/genetic testing typically involves answering three primary questions. Does the information help in arriving at the correct diagnosis—especially in distinguishing benign from malignant tumors? Is prognostic information gained from such testing, or can these data identify certain tumors that will be more apt to respond to specific targeted therapies or treatment strategies? In other words, can the information gleaned from molecular testing assist in analysis of tumors for diagnosis, prognosis, and/or theranostic or predictive response to current and future treatment options and modalities?
 Primary glioblastomas (1 GBM) typically arise de novo in older patients and behave aggressively. Secondary glioblastomas (2 GBM), on the other hand, develop from preceding grade II or III gliomas, typically behave less aggressively, and frequently develop in patients younger than 40 years. Recent advances in surgery, radiation therapy, and chemotherapy have provided only minor improvements in overall clinical outcomes. In time, the development of new molecular markers is expected to improve diagnostic accuracy and prognosis, as well as aid in the clinical management of GBMs.
Primary glioblastomas (1 GBM) typically arise de novo in older patients and behave aggressively. Secondary glioblastomas (2 GBM), on the other hand, develop from preceding grade II or III gliomas, typically behave less aggressively, and frequently develop in patients younger than 40 years. Recent advances in surgery, radiation therapy, and chemotherapy have provided only minor improvements in overall clinical outcomes. In time, the development of new molecular markers is expected to improve diagnostic accuracy and prognosis, as well as aid in the clinical management of GBMs.
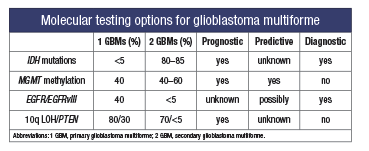
Currently, histologic evaluation remains the gold standard for glioma diagnosis; however, diagnostic difficulty may arise from tumor heterogeneity, ill-defined/overlapping morphologic features, and tumor sampling. Recently, new molecular markers have been developed, some of which have demonstrated diagnostic value, whereas others are useful as prognostic indicators for patient survival and therapeutic response. Overall, 80 percent of astrocytomas have chromosomal abnormalities. GBMs are associated with abnormalities in copy number of chromosomes 7, 9, and 10, especially gains of chromosome 7 and losses of 9p. Oligodendrogliomas are often associated with deletions of 1p and 19q, and tumors with these deletions tend to behave less aggressively and are predictive of a better response to certain chemotherapeutic options. Currently, the most common molecular testing options for high-grade gliomas include IDH1/IDH2 mutation analysis, MGMT methylation analysis, and EGFR and PTEN alteration detection analysis.
- Mutations in one of the two isozymes of isocitrate dehydrogenase (IDH) occur in approximately 40 percent of astrocytomas and oligodendrogliomas but are not seen in nonneoplastic glial tissue, and therefore can be useful in diagnosis when limited sampling is an issue. IDH mutation is associated with young age, a secondary type GBM, and increased overall survival, and it is therefore of both diagnostic and prognostic value. The mutation is generally in the IDH1 gene and is usually a point mutation in exon 4, resulting in a substitution of histidine for arginine (R132H). Sequencing can be done in formalin-fixed paraffin-embedded tissue, and there is an IHC stain for the R132H form of the protein that, when positive, can substitute for sequencing. However, 10 percent of GBMs carrying less common mutations may be missed. Real-time PCR amplification and melting curve analysis was recently reported as another method of detection using fluorescence resonance energy transfer (FRET) probes. This method is faster, less laborious, and more sensitive than sequencing.
- The MGMT gene (O6-methylguanine-DNA methyltranferase) is located at 10q26 and encodes for a DNA repair protein. Epigenetic silencing of this gene by promoter hypermethylation leads to reduced expression of the MGMT protein, which has been shown to result in improved survival in patients with GBM who are treated concurrently with the alkylating drug temozolomide and radiation therapy. The decreased MGMT protein inhibits the cells’ ability to repair alkylated DNA and thus allows alkylating drugs to work more effectively. This marker is therefore prognostic and predictive. Testing methodologies include methylation-specific polymerase chain reaction, real-time PCR, and methylation-specific pyrosequencing. Recently, the extent of MGMT methylation was proposed as a prognostic factor as well.
- EGFR (epidermal growth factor receptor) affects cell proliferation and growth. Activation of EGFR signaling through gene amplification or mutations is found in 30 to 40 percent of primary GBMs. About one-half of GBMs with EGFR amplification contain a mutant variant of the gene (EGFRvIII). Detection of either of these markers is indicative of a high-grade glioma and can be used diagnostically. The prognostic role of these markers is not currently clear. The EGFR signaling pathway is an attractive target for new chemotherapeutic agents such as anti-EGFR tyrosine kinase inhibitors. EGFR amplification can be easily detected by FISH and RT-PCR.
- Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene located on 10q23 and is frequently found in high-grade gliomas. The LOH at 10q is common in primary and secondary GBMs and anaplastic astrocytomas. PTEN mutations are found in 15 to 40 percent of primary GBMs, but they are practically absent in secondary GBMs and other gliomas. Most studies have shown 10q LOH and PTEN mutations as poor prognostic indicators for high-grade gliomas with tumor progression. LOH analysis and FISH are the methods of choice for detection.
In conclusion, some of these molecular markers can be used diagnostically to help the pathologist in glioma classification and grading, especially for tumors with ambiguous histology. Others can be used to estimate prognosis and to predict response to certain therapeutic agents. While none of these tests is ready for prime time or considered standard of care, a working knowledge of these major molecular markers and the molecular diagnostic techniques for their detection is important because their use will undoubtedly increase in routine clinical practice, especially as individualized treatment options are planned.
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773.
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812.
- Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but are rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347.
- Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743–5750.
- Thompson CB. Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med. 2009;360(8):813–815.
- Everhard S, Kaloshi G, Criniere E, et al. MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol. 2006;60(6):740–743.
- Felsberg J, Rapp M, Loeser S, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15(21):6683–6693.
- Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009; 100(12):2235–2241.
- Nikiforova MN, Hamilton, RL. Molecular diagnostics of gliomas. Arch Pathol Lab Med. 2011;135(5):558–568.
- Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16(6):748–754.
- Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024.
Mark P. Burton, MD
Medical Director, Pathology and Medical Laboratory Services
Jackson-Madison County General Hospital Medical Center Laboratory, Jackson, Tenn.
Member, CAP Personalized Health Care Committee Work Group
[hr]
Q. My hospital’s compliance consultants have advised us that pathologists may not order any oncologic prognostic/therapeutic tests that are not needed by the pathologist to establish the pathologic/cytologic diagnosis. As a result, we are requiring written authorization from one of the patient’s treating physicians before we perform testing for hormone receptors, HER2, ALK, EGFR, KRAS, BRAF, microsatellite instability, and DNA mismatch repair.
As the number of these prognostic/therapeutic tests grows, obtaining such authorizations is becoming an increasingly complex and time-consuming process that threatens to interfere with timely and efficient patient care.
Centers for Medicare and Medicaid Services regulations as published in chapter 15 of the Medicare Benefit Policy Manual say a pathologist can order only those tests that are “medically necessary so that a complete and accurate diagnosis can be reported” (80.6.5). All other tests must be ordered by a “treating physician” (80.6, 80.6.1, 80.6.2, 80.6.3, 80.6.4).
Expert guidance would be appreciated.
A. I recognize that there is debate as to whether performing hormone receptor testing is prognostic or diagnostic testing. For purposes of this question, I’ll assume that an auditor considers it to be prognostic.
One of my concerns goes beyond those definitions and is a practical one from the perspective of an attorney who deals with recoupment audits. Regardless of what any manual provision says with respect to the ability of the pathologist to order the test, if there is no documentation of the medical necessity that is convincing to the auditor, then the payment will be denied. In order for the pathologist to document medical necessity from his or her perspective for the particular patient, the medical necessity documentation almost certainly would require recitation of why the test is needed for the pathologist to complete his or her interpretive report (and this is consistent with the context of the manual provision on pathologist ordering). If the test would only be of use to the attending physician, and is not relevant to the interpretation the pathologist is providing, then the auditor could challenge the medical necessity documentation, asking how the pathologist knows that the attending physician believes the additional test would be medically necessary for the attending physician’s diagnosis and treatment of the patient (again, consistent with the context of the manual provision).
In essence, documentation of medical necessity requires that the ordering physician believes that the test/service/etc. (whatever is ordered, whether pathology, imaging, PT, etc.) is medically necessary to the ordering physician’s diagnosis and treatment of the patient. The pathologist can order a test where he or she documents “I believe this is necessary for my interpretive report for the patient.” However, the pathologist should not order a test where he or she doesn’t need the results for the interpretive report but assumes the test results would be useful for the attending physician. That decision should be made by the attending physician.
From this perspective, I believe that the hospital’s guidance and the Medicare manuals are consistent with generally recognized medical necessity principles.
Please note that there are alternatives to establishing medical necessity for the attending physician in addition to obtaining an order from the attending physician. Medical executive committee protocols and standing orders from the attending physician can also be considered.
Jane Pine Wood
Member, McDonald Hopkins LLC, Dennis, Mass.
Dr. Kiechle is medical director of clinical pathology, Memorial Healthcare, Hollywood, Fla. Use the reader service card to submit your inquiries, or address them to Sherrie Rice, CAP TODAY, 325 Waukegan Road, Northfield, IL 60093; srice@cap.org.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
