Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. Are there high-specificity immunohistochemistry stains for diagnosing mesothelioma that differ from those recommended in the “Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group”?
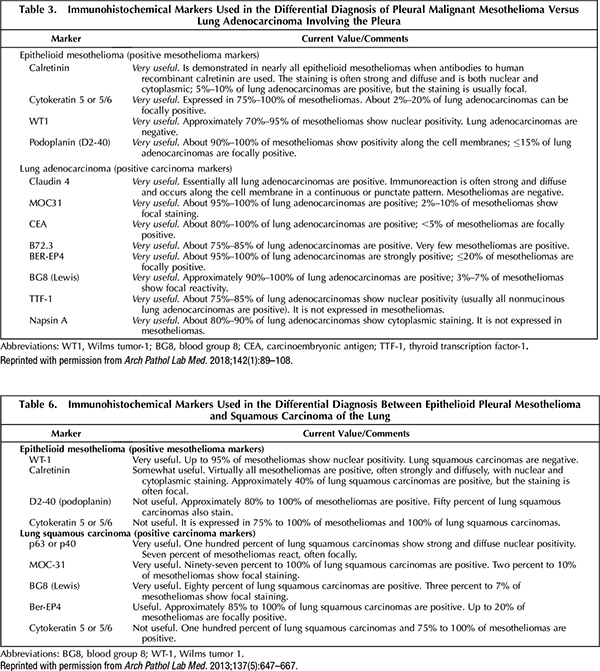
A. June 2020—An updated guideline from the International Mesothelioma Interest Group, published in 2017, did not recommend any new or other markers for mesothelioma. This is seen by comparing table 3 (2017) and table 6 (2012) of the guidelines, as they appeared in the Archives of Pathology & Laboratory Medicine.1,2 However, it is worth noting the addition of claudin 4 as a positive lung carcinoma marker in the 2017 document. Claudin 4 is positive in many carcinoma types but virtually always negative in mesothelioma.While not included in the 2017 update, GATA3 is another potential marker for mesothelioma, and it is expressed in a variety of other tumors as well.3 In addition, BRCA1-associated protein 1 (BAP1) and methylthioadenosine phosphorylase (MTAP) may be useful in distinguishing reactive mesothelial proliferation from malignant mesothelioma.4
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142(1):89–108.
- Husain AN, Colby T, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137(5):647–667.
- Berg KB, Churg A. GATA3 immunohistochemistry for distinguishing sarcomatoid and desmoplastic mesothelioma from sarcomatoid carcinoma of the lung. Am J Surg Pathol. 2017;41(9):1221–1225.
- Kinoshita Y, Hida T, Hamasaki M, et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol. 2018;126(1):54–63.
Dylan V. Miller, MD
Director, Immunostains and Electron
Microscopy Laboratory
Intermountain Laboratory Services
Murray, Utah
Vice Chair, CAP Immunohistochemistry Committee
Anja C. Roden, MD
Medical Director, Immunostains Laboratory
Mayo Clinic
Rochester, Minn.
Member, CAP Immunohistochemistry Committee
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
