Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
Q. What are the steps to validating maximum dilution for certain analytes when the stated manufacturer dilution is not enough?
A. When the stated manufacturer dilution is not sufficient for your laboratory’s needs, it is relatively straightforward to validate an even larger maximum dilution.
A specific example may prove helpful. For test X, the manufacturer’s package insert says the analytic measuring range is five to 100 and the manufacturer has validated, using a 1:100 dilution with normal saline, that one can report values up to 10,000. To report values up to 100,000 (i.e. a dilution of 1:1,000), you would have to take a sample with a value of 100,000 (or higher), make a series of dilutions with the recommended diluent (for example, 1:200, 1:400, 1:800, etc.), and run those diluted samples. You would then correct the measured values for the corresponding dilution and compare them to the expected concentrations, as seen in the table.
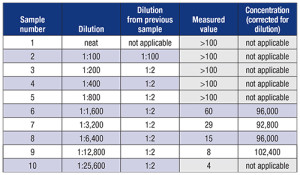 Note in the table that no calculation was done for any measured values outside the analytical measurement range. In this case, samples one through five all yielded values above the AMR, and sample 10 had a value below the AMR. Even though it appears the latter yields the expected value of dilution, it cannot be used because it is outside the AMR.
Note in the table that no calculation was done for any measured values outside the analytical measurement range. In this case, samples one through five all yielded values above the AMR, and sample 10 had a value below the AMR. Even though it appears the latter yields the expected value of dilution, it cannot be used because it is outside the AMR.
One can modify the sequence of dilutions based on the range of the AMR. For example, in the table, one could have probably used serial five- or 10-fold dilutions. Keep in mind that one of the goals of serial dilutions is to have at least two measured values within the AMR; this offers increased confidence in the validity of the results.
In this case, there were four dilutions within the AMR, all of which gave comparable results of approximately 95,000. Based on this sample, it could be inferred that a maximum dilution of 1:12,800 is feasible. You might choose to prepare two separate samples of each dilution to assess the precision of the dilutions themselves. You might also want to repeat the experiment with additional samples to prove it applies to more than a single sample.
The general principle is that if at least two dilutions on a single sample yield results within the AMR, and those results corrected for the degree of dilution are comparable, you have validated the accuracy of the dilution.
- Killeen AA, Long T, Souers R, Styer P, Ventura CB, Klee GG. Verifying performance characteristics of quantitative analytical systems: calibration verification, linearity, and analytical measurement range. Arch Pathol Lab Med. 2014;138(9):1173–1181.
- Jhang JS, Chang CC, Fink DJ, Kroll MH. Evaluation of linearity in the clinical laboratory. Arch Pathol Lab Med. 2004;128(1):44–48.
Gary L. Horowitz, MD, Associate Professor of Pathology, Harvard Medical School, Medical Director of Clinical Chemistry, Beth Israel Deaconess Medical Center, Boston, Chair, CAP Chemistry Resource Committee
[hr]Q. What is considered best practice for verifying platelet-poor plasma for coagulation? Is it necessary to measure platelet counts from 2.7 mL and 1.8 mL tubes? Is annual verification consistent with best practice?
A. The CAP requires that plasma specimens for coagulation testing have a plasma platelet count of less than 10,000/μL. We will briefly review the recommendations for preparation of platelet-poor plasma (PPP) and then discuss best practice for verification of PPP.
Preparation of platelet-poor plasma
Specimen evaluation: Before centrifugation, exclude clot formation by gross observation or, to detect the presence of a clot, remove the cap and insert and then remove two wooden sticks.
Centrifugation: Centrifuge the capped specimen tube at a speed and time required to consistently produce platelet-poor plasma (platelet count
Evaluate specimen post-centrifugation: Perform a visual check for hematocrit and hemolysis. If hemolysis is present or hematocrit is greater than 55 percent, follow your laboratory procedure for high hematocrit and hemolyzed specimens. If a clot is detected, the specimen will have to be rejected.
Aliquoting plasma: When removing the plasma layer, care should be taken to avoid disturbing the cell layers. Remove approximately three-quarters of the top plasma into labeled plastic tubes using a plastic transfer pipette. Pouring off plasma directly from the draw tube will introduce excess cells to the specimen and therefore is not recommended.
Specimen storage: Freeze promptly.
Recommended practice for verification of platelet-poor plasma
To ensure plasma platelet counts are within acceptable limits, the reliability of the centrifugation procedure should be validated every six months and after modification or change of the centrifuge.
At least five sodium citrate tubes should be tested. All steps and precautions described in your laboratory’s standard operating procedure for preparation of platelet-poor plasma should be followed. The platelet counts on all specimens should be tested on a CBC analyzer. All specimens must have a platelet count less than 10,000/μL. If a specimen has a platelet count greater than or equal to 10,000/μL, the centrifuge must be evaluated by clinical engineering and the speed and duration of spin adjusted. All centrifuges used to prepare PPP must be checked by clinical engineering before being put into service and at six-month intervals and should have a label stating clearly the date they were last checked.
Although the CAP and the CLSI do not clearly require separate verification for 2.7 mL and 1.8 mL tubes, in general, one of the factors affecting a particle’s settling velocity in centrifugation is the volume fraction of solids present. Therefore, it may be best practice to verify platelet counts on both 2.7 mL and 1.8 mL tubes.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
