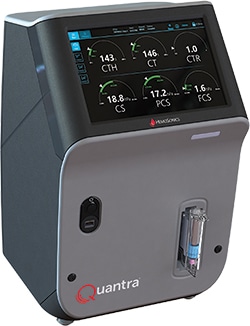
 July 2019—HemoSonics announced that its Quantra Hemostasis Analyzer platform and QPlus cartridge have been granted de novo marketing authorization by the FDA and are available for sale in the United States.
July 2019—HemoSonics announced that its Quantra Hemostasis Analyzer platform and QPlus cartridge have been granted de novo marketing authorization by the FDA and are available for sale in the United States.
The Quantra enables clinicians to assess coagulation status of perioperative patients at the point of care. The system uses a sealed consumable cartridge to run a panel of viscoelastic blood coagulation tests. Hands-on time is less than one minute and results are provided in 15 minutes or less.
Pages: 1 2
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
