Sherrie Rice
June 2022—How race shows up in the medical school curriculum and what to do about it was the focus of a grand rounds by Andrea T. Deyrup, MD, PhD, and Joseph L. Graves Jr., PhD, presented virtually this spring to the University of Minnesota Department of Laboratory Medicine and Pathology, on behalf of the department’s diversity, equity, and inclusion committee.

Dr. Deyrup
Dr. Deyrup, professor of pathology and director of undergraduate medical education for the Department of Pathology at Duke University School of Medicine, is a co-editor and author of Robbins Essential Pathology and the upcoming 11th edition of Robbins & Kumar Basic Pathology. Her Robbins-related comments are about the 10th edition, which she said contains many examples of race-based medicine.
Dr. Graves is a professor of biological sciences at North Carolina Agricultural and Technical State University, author of multiple books on the biology of race, most recently Racism, Not Race: Answers to Frequently Asked Questions (with Alan Goodman; Columbia University Press, 2021), and a consultant for the 11th edition.
In Dr. Deyrup’s examination of the 10th edition, she found more than 35 diseases for which some sort of disparity was mentioned either in prognosis or prevalence based on socially defined race, among them prostate and breast cancer, hypertension, lactase deficiency, diabetes, syphilis, and H. pylori infection. A few examples of how disparities are stated in the text:
- “Four percent of African Americans carry a specific mutation of transthyretin that increases the risk of cardiac amyloidosis in that population over fourfold.”
- “The carrier frequency [of cystic fibrosis] in the United States is 1 in 20 among Caucasians but significantly lower among African Americans, Asians, and Hispanics.”
- “Cholesterol gallstone prevalence approaches 50% to 75% in certain Native American populations (Pima, Hopi, Navajo) whereas pigment stones are rare.”

Dr. Graves
When these and other such statements were written, they were written with the best of intentions, Dr. Deyrup said. “It was believed that these epidemiologic data would help future physicians to develop a broad yet focused differential diagnosis, to order and interpret the appropriate laboratory tests, and then to design the optimal treatment for those patients.”
But it’s not enough, she said, to simply say, “‘We had good intentions.’ We have to ask, ‘Is there any way we’re causing harm?’”—to the patient or to society. “And I believe we are.”
She uses the term “buzzword bingo” to describe how motivated, bright medical students “who are extraordinarily good at multiple-choice questions” are taught simple word associations: white, cystic fibrosis; Black, sickle cell, for example. “And then we reinforce this through our exams, quizzes, clinical vignettes, and cases—even the national boards.”
Posted online in 2015 (crashingresident.com/2015/07/21/rules-for-step-1/) was what Dr. Deyrup describes as “a sardonic, ironic recommendation from a resident about rules for Step 1 for medical students,” one that evokes “a certain amount of horror but also recognition for all of us who are attendings, residents, and fellows. We learned these very associations when we were in medical school, and we also knew they were important for our Step exams.”
It read in part:
Rules for Step 1
Be as Racist as Possible.
- Black people have sarcoidosis, sickle cell disease, keloids, and high blood pressure. Black women definitely have sarcoidosis.
- If a Black person has yellow eyes, the correct answer is “protection from malaria.” Don’t ask why, it just is.
- White people have cystic fibrosis, osteoporosis, weird skin things, and congenital diseases.
- If the person is Jewish, they have a genetic disease and they will probably die from it.
- Asians have a blood disorder called alpha-thalassemia. Japanese people have stomach cancer.
Dr. Deyrup referenced an article by researcher and professor Lundy Braun, PhD, author of a book on pulmonary function tests called Breathing Race Into the Machine, that looked at the UWorld test prep resource, used by medical students preparing to take Step 1 (Ripp K, et al. Teach Learn Med. 2017;29[2]:115–122). They examined the entire database of questions and noted which races were mentioned. They also categorized whether the race or ethnicity was simply descriptive, as in, “It’s a ‘white’ person who has a cold” or a Latin American individual who’s in a motor vehicle accident, or whether it was central, “meaning that knowing that person’s race or ethnicity was key to getting the correct answer,” Dr. Deyrup said.
In 86 percent of the vignettes the person was described as white or Caucasian. “However, this socially defined race makes up only about 62 percent of what we see in America, based on the 2020 census,” Dr. Deyrup said. Most of the time when the term white or Caucasian was used, it was descriptive; it was incidental to arriving at the correct answer. About seven percent of the time, making the association between “white” or Caucasian and the disease process was critical in the item analysis, for example cystic fibrosis.
“However, if a Native American is showing up in your vignette, it is because that person is Native American. It’s not because they were in a motorcycle accident or brushed up against poison ivy; it’s because they have lactase deficiency, gallstones, or diabetes.
“So are we causing harm through buzzword bingo?” Some may say, she said, that these are simply the preclinical years, and surely medical students, as they become residents and attendings, will learn the full spectrum of health and disease and shed this misinformation. “I would counter that by saying there are things I learned 20-plus years ago at the University of Chicago that I did not learn were wrong until I began really looking at race and medicine.”
The next question, she said, is, “Is there a way we’re causing patient harm?”
“I say yes, I think we could be. Say a young Navajo woman presents to an acute care clinic; she has right upper quadrant pain and the physician thinks, Native American. Must be gallstones, and overlooks acute appendicitis or an ectopic pregnancy, either of which could kill the patient.
“You may think this doesn’t happen; we have checks and balances. We have algorithms.” She counters that with a published account titled “The Misuse of Race in Medical Diagnosis,” in which the author recounts a story of a childhood friend who was African American and who wasn’t diagnosed with cystic fibrosis until she was eight years old, despite presenting multiple times with suspicious symptoms (Garcia RS. Pediatrics. 2004;113[5]:1394–1395). This child was diagnosed ultimately by a radiologist who literally couldn’t see the color of her skin but looked at the chest radiograph and said, “Who’s the kid with cystic fibrosis?” Other accounts published recently in JAMA made the point that cystic fibrosis is not a “white” disease, Dr. Deyrup said, and in one article there was an African American who wasn’t diagnosed with cystic fibrosis until he was 54. “So, yes, I think we can be causing patient harm,” she said.
Finally, “Is there a way we’re causing harm to society? Are we, for example, lifting up one population and pushing down another, saying, ‘Ah, bad genes, risky behavior, noncompliant’? Is there a way we are causing some sort of race-based hierarchy?” Dr. Deyrup asks.
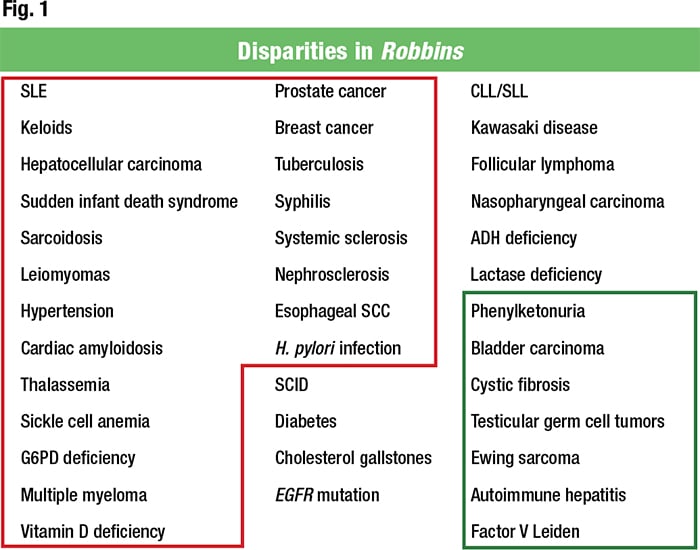
In Fig. 1 she encircles in red the diseases in Robbins for which it is said there’s a worse prognosis or higher incidence in individuals of African descent. For comparison, she encircles in green those for people of European descent. “I think we can say there is this drumming narrative of the diseased African,” Dr. Deyrup said.
“It’s long been known that in anatomically modern humans there is more variability within the so-called races than between them,” he said. “We do have geographically based genetic and physical differences, but any two individuals drawn at random from any two places on the planet share 99.9 percent of their DNA.”
This is enough, he said, to demonstrate that biological race does not exist in modern humans. “However, we do have socially constructed or socially defined race, which arbitrarily uses aspects of morphology, geography, culture, language, religion, or any other thing you can throw into the mix in the service of social dominance hierarchy.” That is why, he said, the book he co-wrote with Alan Goodman is titled Racism, Not Race.
“We make the point in the introduction that it was racism that created our socially defined races, not the underlying biological variation within our species.” This recognition, he said, is beginning to gain traction in modern biomedical research in clinical practice. For example, Ray Merrill’s Introduction to Epidemiology, edition seven, says, “Race is a socially constructed variable based on the idea that some human populations are distinct from others according to external physical characteristics or places of origin.”
However, it says, “Racial or ethnic variations in health-related states or events are explained primarily by exposure or vulnerability to behavioral, psychosocial, material, or environmental risk factors and resources.” Merrill does not say, Dr. Graves points out, that these variations in health-related states are the result of genomic differences between supposed biological races.
This is further indicated by the confusion that exists within, for example, the U.S. census. “The census actually tells you these categories are neither scientific nor anthropological, but they are still being used.”
For example:
- “White—a person having origins in any of the original peoples of Europe, the Middle East, or North Africa.” But then the question becomes, Dr. Graves said, how much of that ancestry makes you white?
- “Black or African American—a person having origins of any of the Black racial groups of Africa.” “Again, how much? And it is important to recognize that Africa contains more genetic variability than all the rest of the world combined.”
- “American Indian or Alaska native—a person having origins in any of the original peoples of North and South America (including Central America) and who maintains tribal affiliation or community attachment.” “This is equally problematic,” Dr. Graves said, “because an American Indian who no longer maintains tribal affiliation no longer classifies in that group. Or what do you do with people like the Black Seminoles who fought slavery and escaped to Florida and became members of the Seminole nation but they have African origin?”
- “Asian—a person having origins in any of the original peoples of the Far East, Southeast Asia, or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, and Vietnam.”
“That illustrates how useless this designation is,” Graves notes.
Dr. Deyrup turned to race in the context of disease and displayed a map delineating genetically based resistance to malaria, with the hemoglobinopathies indicated by color-coded regions (López C, et al. Gene. 2010;467[1–2]:1–12). The hemoglobinopathies are based on evolutionary pressure that selects for these hemoglobinopathies because it offers some protection against malaria, she said.In the United States, sickle cell is considered the African American disease, and large areas of Africa do have sickle cell. It’s also present in Southern Europe and in the Middle East and areas of India. “So we may risk underdiagnosing sickle cell disease in Indian Americans because it is thought of as an African American disease,” Dr. Deyrup said.
“What the map is telling us is that your propensity for this disease—your risk—is not based on geopolitical borders, like the border of your country or even your continent. What it really is based on, in this particular instance, is, were there mosquitoes that carried malaria?” she said, noting an area in Africa where there are no hemoglobinopathies. “Why? The Sahara desert—no water, no mosquitoes, no malaria, no selection pressure, no hemoglobinopathies.” “This is why looking at geographical ancestry can be challenging.”
There is in Central Africa a significant amount of hemoglobin S, and hemoglobin C is primarily in Western Africa. “If this is compared with a map showing the origins of enslaved peoples earlier in the history of our country, it’s clear why there is hemoglobin C and S in the United States.” And this is confirmed by a Howard University study of metropolitan Washington, DC, 2009–2018—part of the university’s sickle cell screening clinic—where it can be seen that while the majority of individuals (86.5 percent) are homozygous for wild-type hemoglobin A (HbAA), there is a significant amount of sickle cell hemoglobin S (about 10 percent) as well as hemoglobin C (about three percent).
“You may be putting this story together and thinking, I can’t think continent of origin or country of origin, but if I start thinking about evolutionary pressure . . . But you can’t just look at somebody’s skin and think that tells you something about where they’re from and what their genetic ancestry is,” Dr. Deyrup said. A 2015 article by Monica Anderson reported that a rising share of the U.S. Black population is foreign born. “They are not the descendants of individuals from chattel slavery.”
Population admixture also has to be considered. Bryc K, et al., reported that the mean amount of European ancestry is about 16 percent for African Americans (defined as Americans descended from enslaved persons) (Bryc K, et al. Am J Hum Genet. 2015;96[1]:37–53). “This is adding additional genetic material into the mix,” Dr. Deyrup said.
Dr. Graves did a deeper dive into population admixture. “When we look at the percentage of European ancestry in persons socially defined as African American, it differs dramatically depending upon the region of the country,” he said. While the mean may be around 16 percent, it varies by state. “For example, in West Virginia it’s northward of 30 percent, and the same is true of Washington State. When we consider people defined as Latino, which is an ethnic group, their percent of European ancestry varies from 65 to 85 percent.” But it’s a tri-admixture that includes African and Amerindian ancestry.“When we look in the Caribbean, for example, on the island of Puerto Rico the average European ancestry is about 72 percent, with 16 percent Amerindian ancestry and 12 percent African ancestry. And there are more Puerto Ricans in the continental United States than there are on the island of Puerto Rico.” Mexican Americans are on average about 51 percent Amerindian in ancestry, 46 percent European, but only three percent African.
“When we look at some of the South American nations like Colombia, they have an admixture portfolio that looks something like between that of Puerto Rico and Mexico,” Dr. Graves said. “And if we look at Peruvians, they are 80 percent Amerindian by ancestry and 18 percent European, with a small percentage of African ancestry.” But even within the nation of Colombia, there can be dramatic differences in different parts of the country.
In the city of Medellín, for example, you see the tri-admixture looking very much like the national average. But in the neighboring state of Chocó, which was a center of plantation agriculture and had a large number of enslaved Africans, their tri-admixture looks very much like African Americans, although it has a much higher percentage of Native American ancestry.
Dr. Deyrup displayed a chart of melanoma subtype incidence by socially defined race (non Hispanic white, Black, Asian/Pacific Islander, Hispanic white) (Huang K, et al. J Surg Res. 2020;251:329–339). “And this is using the SEER data,” for which it’s important to recognize the limitations, she said.Melanoma risk is based on the amount of pigmentation in the skin. “We know melanin is protective; we teach this to our medical students. And there is this belief that melanoma is incredibly uncommon in patients of African descent.”
“We’ll often talk socially about Black and brown people and Hispanics are included in that. But when we’re talking about SEER data, because of political ways of looking at people, they are considered white. Definitely a disconnect. And part of the issue I’m pointing out here is that we know that superficial spreading melanoma is something we tell our medical students to look for in blonde and red-haired patients. But we’re missing it in our African American patients. They are presenting now at a later stage. So these labels we’re using have a tremendous impact on patients.”
It’s clear, she said, that race is not a biologically useful construct. Why then are there racial disparities in health and disease?
There are some genetic bases for these disparities, and she refers to these as intrinsic causes of disparities, a founder effect perhaps or a population bottleneck. This could be severe combined immunodeficiency in the Navajo Indians and Tay-Sachs in the Ashkenazi Jewish population, groups that were decimated in the American genocide of the Native Americans and in the Holocaust, respectively. “What we’re looking at here are not races; they are specific populations.”
Another cause of allele frequency differences would be endogamy, due to religion, caste, or geography. Here, too, it is not a race; it is a population. Finally, there is selective advantage: selective sickle cell or the other hemoglobinopathies—again, related to geography, not races.
While much time is spent focusing on allele frequencies and doing genomewide association screening, the “primary mover and shaker” of health disparities, particularly in the U.S., she said, are the extrinsic causes: poverty, stress, access to care, living conditions, food access, neighborhood, culture, exercise, education, racism (individual, institutional, systemic), and others. Many of these are related and all, she said, are shadowed by individual, institutional, and systemic racism.
She describes three types of racism, as explained at dismantlingracism.org.
“Personal racism is what I once thought most racism was: men in white robes burning crosses. And we are currently seeing a surge in personal racism in recent acts of anti-Black terrorism. As a country we have come to recognize that institutional and systemic racism also have tremendous impacts and support and are supported by personal racism. Institutional racism would be considered the policies and practices of institutions like universities or hospitals that include and serve and financially resource white people.” A medical example of this would be that when the first phenylketonuria screening testing was begun, in that first clinic, only white children were screened because of the belief that only white children got PKU, “which we know is not true.”
Cultural or systemic racism is defined as beliefs, values, and norms that uplift and validate and protect white populations at the expense of people and communities of color. “Examples of this would be that our society doesn’t believe in universal health care, which has a tremendous impact on health disparities. Also, our government practiced redlining, which made it more challenging for African Americans to get mortgages and own homes, creating multi-generational poverty, which, as we know, is associated with health disparities.”
She looks at the extrinsic factors in the context of disease. For hysterectomy, for example, there are two large categories: the open hysterectomy (sizable abdominal incision the healing from which can have a significant amount of morbidity), and the minimally invasive hysterectomy (laparoscopic or vaginal). Studies of women eligible for the latter—that is, a uterine size compatible with either approach—have found that eligible Black, Hispanic, and publicly insured women were least likely to receive minimally invasive techniques compared with white women (Pollack LM, et al. J Minim Invasive Gynecol. 2020;27[5]:1167–1177.e2; Price JT, et al. Am J Obstet Gynecol. 2017;217[5]:572.e1–572.e10). “I would say the factors involved in this would be systemic racism based on the criterion of public insurance. And institutional racism because the hospitals that serve these populations had fewer surgeons that could perform minimally invasive techniques. And then there is poverty, education, access to care, and health insurance.”
Once Dr. Deyrup compiled the long list of diseases for which there are disparities in Robbins, she did a deep dive into the literature and generated a lengthy paper that she is sharing with her medical students and on the basis of which she is creating videos (pathologycentral.org). “Part of this work is so that we can change it in the upcoming edition of Robbins,” she said.“We need to look at the data so we can address how we move forward.” People could say race is problematic and suggest removing all mentions of race from all textbooks, she says. While she thinks the science will support that for the vast majority of cases, she predicts the pushback from systemic racism would overwhelm it. So, to move forward, she and colleagues have done deep dives into the science.
Keloid is an example. The following is in the 10th edition of Robbins: “Certain individuals seem to be predisposed to keloid formation, particularly those of African descent (italics added).” Dr. Deyrup recommended to the editor overseeing the relevant chapter that the italicized phrase be removed, and the editor referred her to the following statement in UpToDate: “Keloids have been reported in 5 to 16 percent of individuals of Hispanic and African ancestry [5].” Because she had written the paper on disparities, she had not only read UpToDate (“always a good place to start,” she says) but also reference five (and all other references on keloid), in which a 4.5 to 16 percent statistic appears, as well as the following: “Overall, the risk of developing keloids is approximately 15 times higher in dark-skinned individuals compared with whites” (Robles DT, et al. Clin Dermatol. 2007;25[1]:26–32).
“Fifteen times is pretty significant from a statistical point of view,” she said, and she had seen it in other articles. The primary source appeared to be a 2001 article by Brissett AE, et al., which said, “In a review of 175 cases of keloids from various races, Alhady and Sivanantharajah found that keloids were 15 times more likely to occur in darker-skinned individuals” (Brissett AE. Facial Plast Surg. 2001;17[4]:263–272).
“With the tenacity of a bulldog,” she said, she went after that 1969 article, which was research done in Malaysia. It says, “. . . the relatively fair-skinned Chinese appear to be slightly more prone [2.4–3.3 fold increase] to keloids than the dark-skinned Indians and Malays” (Alhady SM, et al. Plast Reconstr Surg. 1969;44[6]:564–566).
“So the arrow is pointing in the wrong direction,” Dr. Deyrup said, “because what the authors actually found was that the relatively fair-skinned Chinese were slightly more prone than the dark-skinned Indians and Malays.”
She did the math herself and the number is wrong too: “It’s about a two- to threefold increase,” she said. She presented all of her findings to the editor and this statement will appear in the 11th edition: “Keloid formation seems to be an individual predisposition.”
“Which it is,” she said. (The full story of the deep dive, which explains the origins of and problems with the 4.5 or five percent and 16 percent statistics, is at pathologycentral.org.)
She cites a 1980 article by an African American plastic surgeon who found in his practice that African Americans were not prone to keloids and said his patients were reluctant to have elective surgery because they feared a keloid. “He said, ‘You can just look at the patient’s skin, because most of us have had some sort of abrasion, piercing, laceration, or surgery. And if you have a tendency to get a keloid, you’ll have a keloid. So I don’t need to look at the color of your skin to project whether you’ll have a keloid.’”
For children, who have likely had less skin trauma than adults, a close family history would be the right approach, Dr. Deyrup said. “And we can use that approach not just for keloids but for all diseases because this will tell you more about that person’s geographic ancestry and genetic background than anything related to socially defined race.”
Is a focus on disparities in medicine data or distraction? she asks. “It can be data if we take information, for example, looking at severe combined immunodeficiency in the Navajo and develop a newborn screening program so those patients can get the stem cell transplants they need.“It’s data if we are recognizing that an African American who’s a man and is in his 60s and it looks like he has hypertensive cardiomyopathy but no hypertension—let’s look to see if he has cardiac amyloidosis, for which we have a pharmaceutical treatment.
“It’s a distraction if we do what we’ve been doing for generations and saying white equals cystic fibrosis and Black equals sickle cell disease.”
What should educators, as physicians, do to make disparities and their focus on those disparities data and not distraction?
“First we have to be inclusive. We need to have thoughtful, mindful analysis of the material driven by data, and we need to provide context.” For inclusivity, it’s important to build a team, she said, “people who can help you see the many different perspectives necessary to do this work.” As you’re building your team, start off slowly and gently, she said, and keep in mind you have to do your own work. “It’s critical for those of us who want to be allies, co-conspirators, that we do our work, that we come ready to work. And as you develop your skills, invite in people who may challenge you more, who help you grow into this role.”
Include the medical students and the residents, Dr. Deyrup urges. “Our younger physicians in training are thinking about this, and it’s often challenging for us as professors to reach out, to show humility and vulnerability. But we have to be vulnerable. We have to be transparent. We have to reach out because this is how we learn.” This is what she did for the 11th edition of Robbins. “I emailed the Duke medical students, ‘If you see anything in the 10th edition that you think is inappropriate, let me know. I put up a Google Doc; it’s completely anonymous.’”
Be inclusive with images. At Duke, since 2016, she, influenced by her colleague Kenyon Railey, MD, removed all mentions of race and ethnicity from her clinical vignettes and in the questions in teaching cases. But there are still images, and although there’s a nice mix of patients of different skin types, it’s not enough, she said. “We can’t just say, ‘And here is the canonical basal cell carcinoma on this person of European descent.’ We now include in our website an alternative image of a basal cell carcinoma in an individual with a darker skin tone.” They’re doing this, too, in Robbins, and not just with the images but the text. “How many times have you read psoriasis is characterized by salmon-pink plaques? They’re salmon-pink if you have lightly pigmented skin, but they’re not if you have darker skin, in which they often appear hyperpigmented. So we have to look everywhere for this. It’s very sneaky how systemic racism has infiltrated everything in medicine.”
“Question everything,” she advises. “You have to keep looking and knocking it down.”
As far as how it can be done appropriately, she advises using the right terminology and providing context. The 10th edition of Robbins says the following: “Approximately 2% to 15% of whites carry a specific factor V mutation (called the Leiden mutation) . . .”
“In the 11th edition we’re not using colors because if you use white, that opens you up to using brown, Black, yellow, and red and nobody wants to do that. In the case of factor V Leiden, we know this is a mutation, it’s genetic, so we want to emphasize ancestry. It will say the ‘mutation is seen in approximately 2% to 15% of individuals of European ancestry.’ But we don’t want this to be code word for white. We don’t want this to be, ‘Hey, medical students, when Deyrup says European ancestry, she means white.’ We need to give them a further nudge and say it can be ‘present to varying degrees in other American groups, largely due to population admixture.’” This is particularly relevant for factor V Leiden because heterozygotes can be prothrombotic as well.
“We need to be sure people are not checking off a little box and moving on.”
Sherrie Rice is editor of CAP TODAY.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
