Anne Paxton
December 2023—Hoi-Ying Elsie Yu, PhD, D(ABCC), isn’t new to workflow optimization. As system director of chemistry, point-of-care testing, and preanalytics for Geisinger Medical Center in Danville, Pa., for the past decade, she has undertaken initiatives to maximize efficiency in complicated parts of the laboratory whenever she can.
“No matter what, there’s always an opportunity to further optimize,” says Dr. Yu, who also serves as Geisinger’s director of clinical pathology informatics.

Dr. Yu
Through a partnership with Thermo Fisher Scientific, Geisinger’s immunology laboratory, which has been under Dr. Yu’s direction for the past five years, used workflow analysis to consolidate its allergy and autoimmune testing. Dr. Yu and her team replaced six allergy and autoimmune testing platforms (two Dynex DSX, two Werfen Bio-Flash, two Siemens Immulite 2000) with three (two Thermo Fisher Phadia 250 systems and one Phadia 1000 running portfolios of autoimmune and allergy tests). The laboratory had one Zeus IFA before and after the consolidation.
Between May 2022 and June 2023, the lab team saw the following:
- A reduction in staffing needs by one full-time-equivalent, time that was redirected to other work.
- A 69 percent reduction in total manual labor time, saving approximately $40,000, and 67 percent less time spent managing inventory.
- A savings of 90 square feet of laboratory space.
- Standardization of practices by reducing four methods to two.
- Reduced medical technologist time spent walking back and forth during daily operations, and 80 percent fewer interfaces with PCs.
- 71 percent fewer calibrations and 59 percent less reagent storage space utilization.
- The capacity for a 77 percent increase in test volume.
These outcomes were reported this summer in a poster presented at the Association for Diagnostics and Laboratory Medicine meeting. In recent interviews with CAP TODAY, Dr. Yu and Jessica Murphy, MLS(ASCP), technical laboratory educator for Thermo Fisher Scientific, elaborated on the instrument consolidation project.
Prior to the instruments upgrade, Geisinger’s immunology laboratory was using seven platforms for testing, Dr. Yu says.
“The DSX, the Immulite, and the Bio-Flash had been acquired over a number of years, in many cases to fill a specific clinical need and/or because of a favorable price point. I inherited them.”
Nexus, which provides workplace consulting, conducted the interviews of immunology laboratory staff for the consolidation study. Thermo Fisher’s workflow analysis team assisted with the study as a service it offers to customers at no charge, Murphy says.
In autoimmune disease testing, she notes, “it’s very much apples to oranges when you’re comparing different methods, and that’s well known in the lab world. One of my main roles at Thermo Fisher is educating the laboratory on how to utilize our Phadia 250 and Phadia 1000 platforms and how our technology compares with other methodologies within the lab, and helping explain the results of correlation studies.” Dr. Yu herself worked with clinicians to help them understand the differences in the results. “Our method comparison studies were reviewed with clinicians prior to go live,” she says.
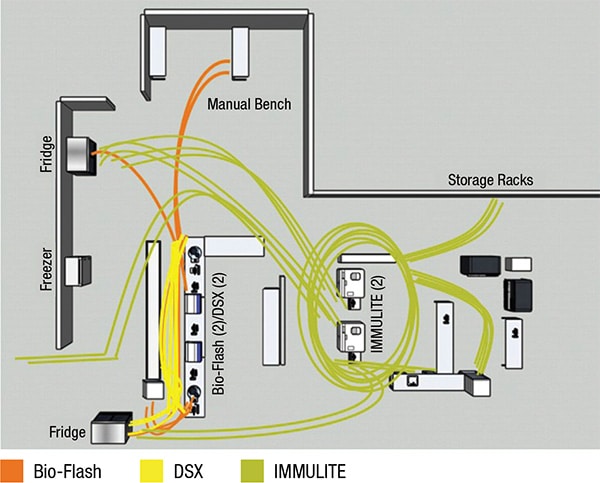
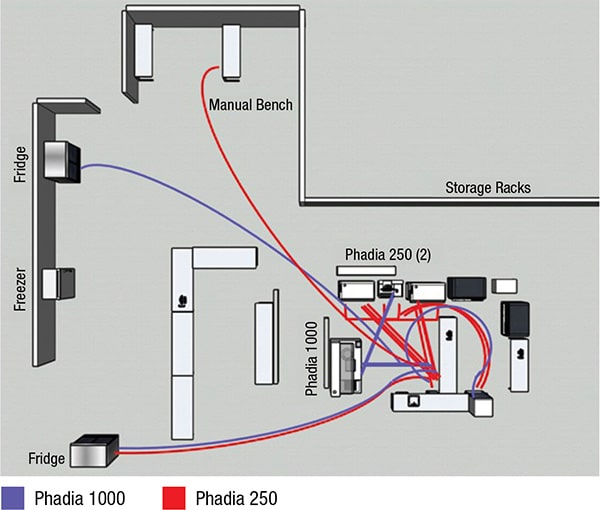
For the study’s pre-workflow and post-workflow analyses, Thermo Fisher and Nexus did interviews, collected operational data through direct workflow observations, and performed time and motion studies in the immunology laboratory. Murphy and Dr. Yu say they see the workflow optimization process as different from Lean or Six Sigma process improvement, which focuses more on eliminating waste and improving business processes.
“This particular workflow didn’t check all the Six Sigma boxes,” Murphy says. “We made this a more customized workflow analysis for Geisinger based on their needs and what they wanted to see in the data and the outcomes.”
“As we know,” she says, “labs are being stretched thinner and thinner. And that’s a big reason why we have been completing more and more of these workflow analyses.”
Reducing the physical steps of technologists was one effect of the consolidation. Different technologists may do things differently, Dr. Yu says, and there may be reasons for that. “But you don’t know how crazy and nonstandardized the workflow is” without seeing diagrams. “It’s eye-opening for someone in a management position who wants to understand whether there is room for improvement.”
With the pre-workflow spaghetti diagram highlighting the pathways of technologist steps to the manual bench (see diagrams), the laboratory saw a way to reduce those steps in particular, primarily through a change in reflex testing, Dr. Yu says. “The major thing that happened was we limited the ability for providers to order an IFA [indirect fluorescent antibody assay].”
If the antinuclear antibody screen was positive, they usually reflexed to IFA. “But now, if the screening is positive, we reflex to quantification of the antibody. And then we don’t have to do an IFA,” Dr. Yu explains. Instead, the IFA testing is more likely to be used by specialists such as rheumatologists or hepatologists who do order the test.
The lower volume of IFA testing explains much of the immunology lab’s ability to reduce staffing needs by one FTE. “The IFA test is labor-intensive with the slide preparation and reading. Result interpretation is rather subjective and semiquantitative at best,” Dr. Yu says, noting the more automated options for IFA can be costly. “Phadia immunology testing offers quantitative, semiquantitative, and qualitative results, dependent on assay, and the instruments are much more automated.”
Then, too, the three Phadia instruments are sitting in the same corner, she says, “so I can have one person managing three instruments, versus before when they were in different corners.”

Murphy
Says Murphy: “Now, as before, the immunology lab requires two and a half full-time technologists. The manual bench didn’t necessarily require a full-time position when they were reading the manual IFAs and ANA assays, but it did require two full-time technologists to run the six other instruments, and now it takes only one full-time technologist to run the Phadia bench. The lab was also able to expand its test menu, increasing volumes and increasing revenue within the lab.”
The increase in immunology lab test volume for which additional capacity was needed came largely from two developments, Dr. Yu says. One was growth stemming from Geisinger’s regional expansion. “We typically have seen organic growth because our territory got bigger and so did the number of patients we serve.” Some regional hospitals closed in the wake of the pandemic, “so we absorbed those patients and we’ve grown our facility to make it attract more patients.” That has driven increases in microbiology, chemistry, and hematology testing at Geisinger as well as in immunology.
Profile optimization in allergy testing has also driven volume. In Dr. Yu’s laboratory, the autoimmune antibody test volume didn’t rise that much. “The increase was mostly in the allergy testing, which was over 100 percent higher,” in part due to expanded allergen testing, Dr. Yu says.
Allergy testing profiles are arranged typically based on clinical presentations and geographical location (for example, respiratory symptoms) or likelihood of allergens (for example, mold). “We used to have what we called the Northeast profile; it’s the Northeast respiratory profile. When we looked back, we realized it doesn’t have a lot of common allergens that should be in the profile. So we added allergens to make sure we covered more of the common trees and grasses.”
A small profile is still available. “But we have a bigger profile so that if a patient does have respiratory symptoms, the allergists can order a panel that will cover most allergens.”
An added benefit for the immunology laboratory, with its seasonal fluctuations in allergy test volume in spring and fall, is that the Phadia 1000 can provide results quickly, Murphy says, so that technologists can still complete tests within an eight-hour shift—and if the volume is still too large, they have the two Phadia 250 instruments to fall back on.
Dr. Yu says she and her staff started looking at instruments about two years prior to their having the money to purchase.
Post-consolidation, “hospital administrators always want to see the money they gave us paid back.” For her laboratory, the pre- and post-consolidation workflow analyses have been helpful in proving savings as a return on investment in the new instruments.
Larger-volume labs and community hospital labs alike could benefit from the Geisinger workflow optimization study, Murphy says. “I don’t feel there’s one definition of workflow analysis, and there are a lot of different needs for it. This specific study may be more for larger-volume laboratories or even reference laboratories. But for smaller labs, different types of workflow analysis can bring value as well, whether it’s where the instruments should be put, how the instruments should be run, whether they should just be run on Mondays, Wednesdays, and Fridays, whether to do combined runs of allergy and autoimmune testing, or what the most efficient way is to manage reagent usage.”
Anne Paxton is a writer and attorney in Seattle.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
